DermBiont has raised $35.2m in a Series B financing round to push two of its dermatology candidates through Phase II trials.
The funding consists of $27.1m raised through a Series B first close and $8.1m in converting outstanding notes, based on a 24 October press release. The Series B was led by new investor Double Point Ventures with support from existing investors, including Viking Global Investors, Civilization Ventures, and Olive Tree Capital.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
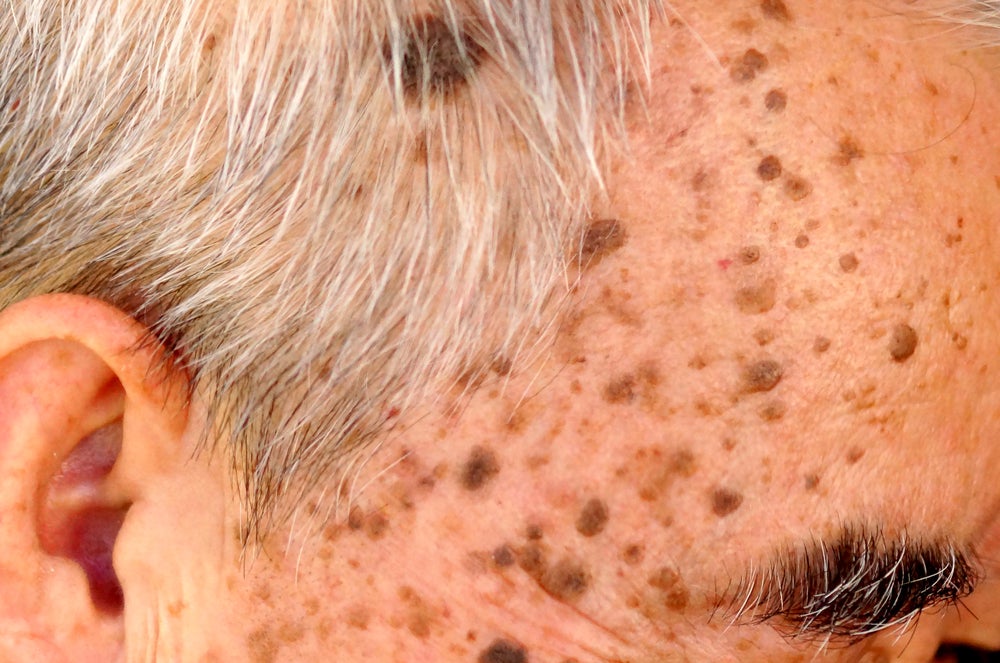
In December 2021, the company raised $28m in a Series A2 financing roundDermBiont stated that the new funds will go towards advancing its two drugs in development – SM-020 for the treatment of seborrheic keratoses (SKs) and SM-030 for the treatment of melasma and other hyperpigmentation disorders of the skin.
DermBiont acquired the drugs as assets when it acquired SeylanMED in October 2020. SM-020 is an AKT kinase inhibitor while SM-030 is a PKC-beta inhibitor.
DermBiont anticipates having Phase II meetings with the US Food and Drug Administration (FDA) for both programmes in H2 2024. The company said it expects the funds to last until the end of these FDA discussions.
SKs are currently treated using procedures such as cryotherapy and shave excision. In December 2017, the FDA approved Aclaris Therapeutics’ hydrogen peroxide topical solution Eskata for use in raised SKs.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn March 2023, DermBiont announced positive results from a Phase II proof of concept trial investigating SM-020 for SK treatment. The trial met its primary endpoint of a one-point improvement in the physician’s lesion assessment (PLA) score.
SM-020 will be further investigated in patients with SKs across three Phase II trials. SM-020 1% gel will be given twice daily for 28 days to 60 subjects with SKs in a randomised, double-blind, vehicle-controlled Phase IIb trial.
In a separate Phase IIb trial, the same formulation and regimen will be administered to ten subjects with dermatosis papulosa nigra – a subtype of SKs. DermBiont will also use SM-020 0.1% gel for 28-56 days in an open-label clinical extension in 25 additional subjects with SKs.
For SM-030 gel, DermBiont will test the drug at three different doses for 12 weeks in a randomised, observer-blinded, placebo-controlled Phase IIb trial in 138 subjects with melasma. In May 2023, the company announced results demonstrating the candidate achieved safety and efficacy endpoints in a Phase II trial for solar lentigos and photoinduced pigmentation.
DermBiont CEO Karl Beutner said in a statement: “The addition of Double Point Ventures as a major backer alongside continued financial and strategic support from Viking Global Investors and other investors allows us to accelerate the development of our pipeline of novel topical therapeutics for the treatment of the number one and number three most frequently diagnosed disorders of the skin by dermatologists in the US.”




