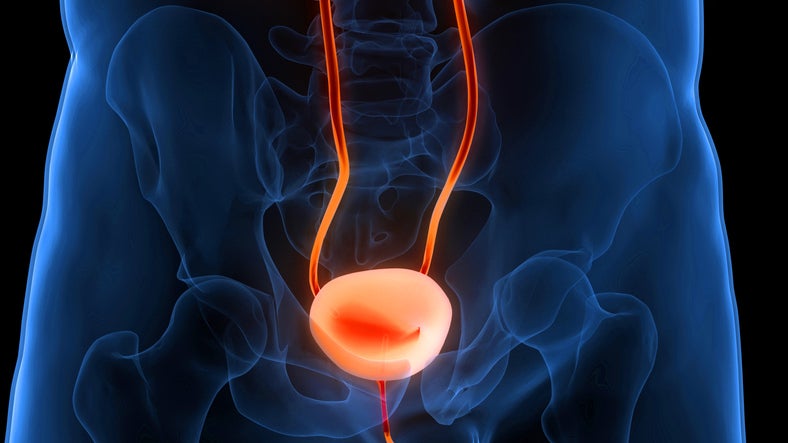
The US Food and Drug Administration (FDA) has granted fast track designation and breakthrough designation to CG Oncology’s cretostimogene grenadenorepvec to treat patients with Bacillus Calmette-Guerin (BCG)-unresponsive non-muscle invasive bladder cancer (NMIBC).
The FDA decision is based on results from an interim analysis of the ongoing Phase III BOND-003 clinical trial, which enrols 110 patients. The interim data from 66 patients demonstrated clinical benefit and showed cretostimogene grenadenorepvec is well tolerated, with a complete response rate of 75.7%. The study has an estimated completion date of July 2025.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The Californian company showcased the interim data at the Annual Meeting of the Society of Urological Oncology (SUO), which took place in Washington on 30 November 2023.
CG is also running several combination clinical trials, including a Phase II study of cretostimogene grenadenorepvec in combination with Merck (MSD)’s Keytruda (pembrolizumab) in patients with NMIBC, unresponsive to BCG.
The BCG vaccine is used as a treatment for preventing or delaying tumour recurrence following high-grade NMIBC. Cretostimogene grenadenorepvec is an oncolytic virus therapy engineered to secrete an immune-stimulating hormone.
CG Oncology raised $105m this year in a funding round jointly led by Foresite Capital and TCGX to advance bladder cancer programmes such as cretostimogene grenadenorepvec.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIf successful, cretostimogene grenadenorepvec will be entering the treatment landscape for NMIBC, which notably includes Keytruda. The FDA approved Keytruda as a treatment for NMIBC in 2020, with the drug making $20.9bn globally last year, as per MSD’s full-year 2022 financial results.
In the announcement accompanying the designations, CG Oncology president and chief operating officer Ambaw Bellete said: “Receiving both FDA fast track and breakthrough therapy designation is an important milestone in the development of cretostimogene grenadenorepvec and for patients with bladder cancer who urgently need more therapeutic options.”
According to a report on GlobalData’s Pharma Intelligence Center, the bladder cancer market in the 7MM (US, France, Germany, Italy, Spain, UK, and Japan) is forecast to be worth $5.59bn.
GlobalData is the parent company of Pharmaceutical Technology.




