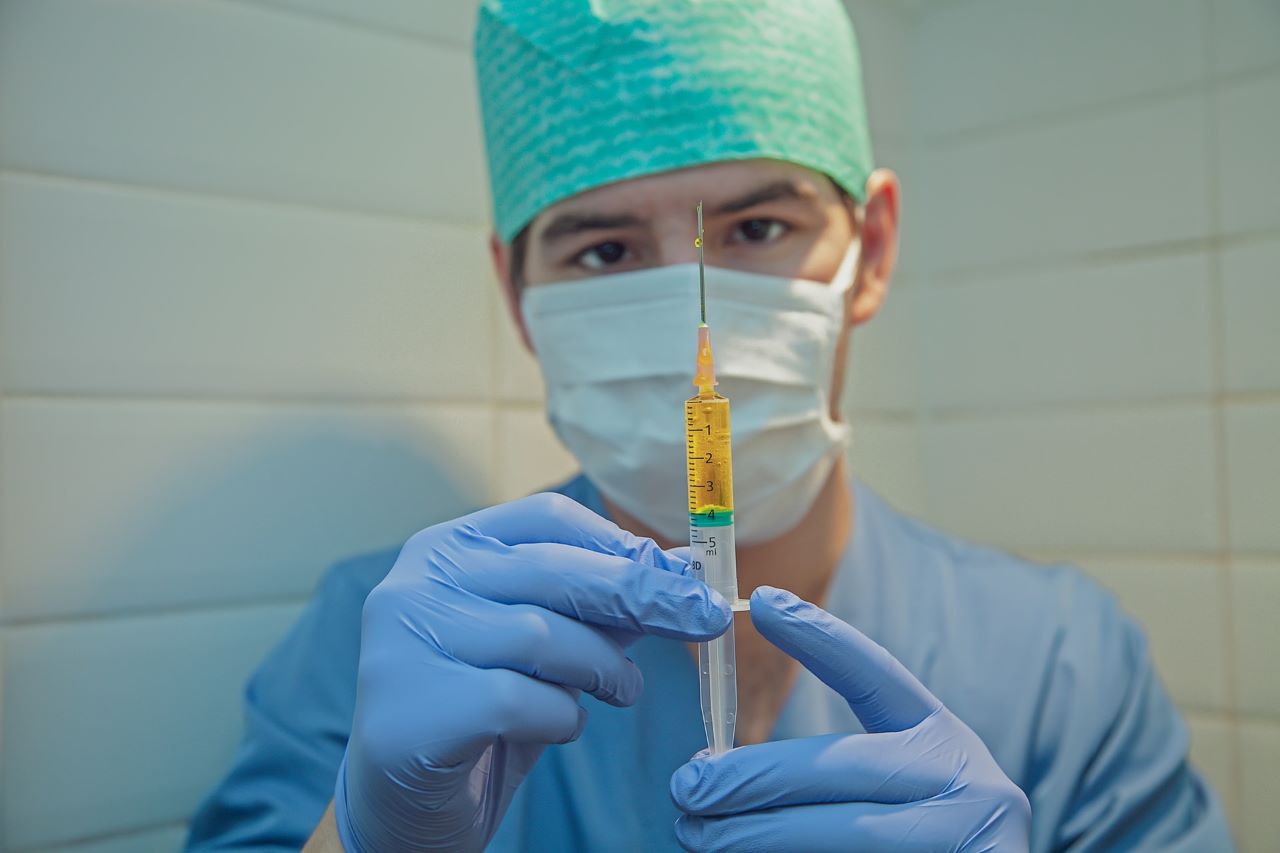
Indian firm Bharat Biotech and biopharmaceutical company Ocugen have signed a binding letter of intent (LoI) to co-develop the former’s Covid-19 vaccine candidate, COVAXIN, for the US market.
COVAXIN is an advanced stage whole-viron inactivated vaccine candidate.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
In the Phase I and II clinical trials in India, the vaccine has been analysed in around 1,000 subjects, with promising safety and immunogenicity data.
It is currently in Phase III clinical trials with 26,000 participants in the country.
According to the LoI, Ocugen will have US rights to COVAXIN and handle clinical development, registration and commercialisation for the US market.
Bharat Biotech and Ocugen have begun working together and will decide on details of the definitive agreement soon.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThis collaboration leverages Ocugen’s vaccine expertise, and its R&D and regulatory capabilities in the US.
Ocugen chairman, CEO and co-founder Dr Shankar Musunuri said: “We are delighted to collaborate with Bharat Biotech to potentially bring COVAXIN to the US market.
“We have been very pleased with the safety and immunogenicity demonstrated by the Phase I and Phase II trials of COVAXIN and are encouraged with the progress of the Phase III trials in India.”
For the development of the vaccine, Ocugen has assembled a Vaccine Scientific Advisory Board featuring academic and industry professionals to analyse the clinical and regulatory path to approval in the US market.
Bharat Biotech chairman and MD Dr Krishna Ella said: “COVAXIN has garnered interest from several countries worldwide for supplies and introduction and we are excited to collaborate with Ocugen to bring it to the US market.”
Last week, Bharat Biotech reported interim results from the Phase I clinical trial that showed COVAXIN induced a robust immune response with no serious adverse effects.




