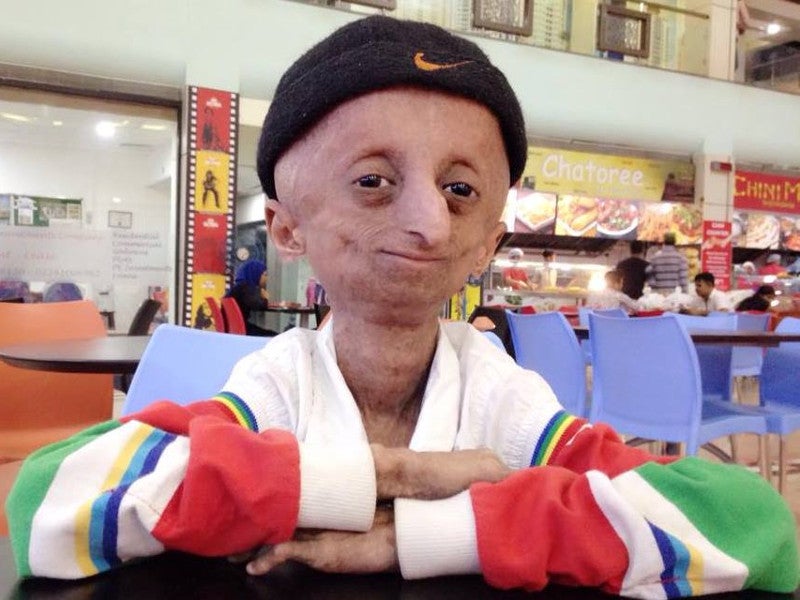
Progeria and its causes and symptoms
Hutchinson-Gilford Progeria Syndrome (HGPS), commonly known as progeria, is a fatal and ultra-rare genetic condition that affects approximately 400 children worldwide. Progeria patients die early due to conditions such as atherosclerosis, skeletal dysplasia, and stroke.
Progeria and the most progeroid laminopathies are caused due to a mutation in the lamin A/C (LMNA) gene, which encodes for lamin A protein. The progeria-causing gene was discovered by the Progeria Research Foundation (PRF) in 2003.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The lamin A protein is essential for the stability and functioning of the nucleus in the cell. An abnormal quantity of lamin A causes overproduction of a defective farnesylated protein called progerin, which is believed to cause premature ageing in children.
Progerin renders them typical facies such as prominent eyes, scalp veins, loss of hair, scleroderma-like skin, and stunted growth.

Development history and in-licensing of lonafarnib by Eiger
Originally developed by Merck, lonafarnib was initially studied as a potential cancer therapy, but later repurposed for clinical studies in progeria patients. It has also shown positive outcomes in patients with chronic hepatitis delta viral (HDV) infection.
Eiger in-licensed the drug from Merck with the aim of developing it to treat HDV infection, in 2010. Eiger expanded the licence agreement in May 2018 for worldwide rights for regulatory submission, commercialisation, and distribution of lonafarnib for progeria treatment.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataMerck and Eiger Biopharma have been supplying lonafarnib to the research community for clinical studies under a licence agreement.
How Lonafarnib works
Lonafarnib is a farnesyltransferase inhibitor that blocks farnesylation, an important process for the functioning of the lamin A protein and progerin.
First-ever clinical study on progeria with lonafarnib
Lonafarnib was investigated for progeria for the first time in a phase two, open-label clinical study in Boston Children’s Hospital in Massachusetts, US, in 2007. The study enrolled 29 patients from 16 countries with HGPS and progeroid laminopathies, out of which 26 had classic HGPS.
The patients with classic HGPS received oral lonafarnib twice daily for two years. The rate of weight gain by patients was set as the primary endpoint of the study. Lonafarnib was well-tolerated by all the patients and none of them exited the trial due to toxicity.
Out of the 25 patients who completed at least two years receiving oral lonafarnib, nine patients experienced equal to or more than a 50% increase in the rate of weight gain, while ten remained stable. Another six patients experienced a decline of 50% or higher in the rate of weight gain.
Vascular stiffness, which increases the risk of fatal cardiovascular events such as myocardial infarction and stroke, decreased by 35% in patients administered with lonafarnib. Vascular stiffness is considered as an independent predictor of mortality due to coronary events in the general population.
The treatment also improved bone structure formation by improving bone mineral density, and the hearing abilities of the patients in both ears. The treatment demonstrated improvement in all patients in one or more outcomes.
Lonafarnib still the prominently investigated drug for progeria
Out of the six interventional clinical trials that have been initiated on progeria till date, Lonafarnib is utilised in four, either as a monotherapy or in combination with other drugs.
Lonafarnib significantly lowered the mortality rate in patients by 80% in one of the clinical trials approved by the Rhode Island Hospital Institutional Review Board, which evaluated the safety and efficacy of lonafarnib in more than 204 patients.
Five out of the 43 patients treated with lonafarnib died, compared to 21 in the untreated group during the trial, including 5.3 years of the follow-up period. The drug increased the lifespan of children with progeria by at least one year and six months.

Children treated with lonafarnib also demonstrated improved neurologic status. The prevalence of stroke, transient ischemic attack (TIA) and seizures was reduced, as well as the frequency of headaches was lower than the rates before the trial.
Efficacy of lonafarnib as a combination therapy
Lonafarnib is being evaluated in combination with other drugs such as zoledronic acid (treats bone diseases), pravastatin (cardiovascular drug), and everolimus (chemotherapy drug).
A triple-combination therapy of lonafarnib, zoledronic acid and pravastatin in 37 patients demonstrated benefits such as improved bone mineral density in comparison to lonafarnib alone. No additional cardiovascular benefit was observed with the addition of pravastatin and zoledronic acid, compared to lonafarnib alone, in the trial.
Another clinical study was initiated in March 2019 in Bundang CHA Hospital, Korea, that enrolled two progeria patients to evaluate the efficacy and safety of umbilical cord blood therapy.
Progeria treatment advances: Where lonafarnib currently stands for treatment?
Over the last decade, lonafarnib has prolonged the survival of progeria patients along with one or more benefits, including weight gain, improved bone structure and hearing ability, as well as a reduction in vascular stiffness.
Eiger is collaborating with Progeria Research Foundation, the only non-profit organisation for progeria research, under the priority review voucher programme, and plans to submit a new drug application (NDA) to the FDA in 2019.
The company also plans to submit marketing authorisation applications to the European Medicines Agency in 2019.
If approved, lonafarnib would significantly prolong survival and improve the life of progeria patients.




