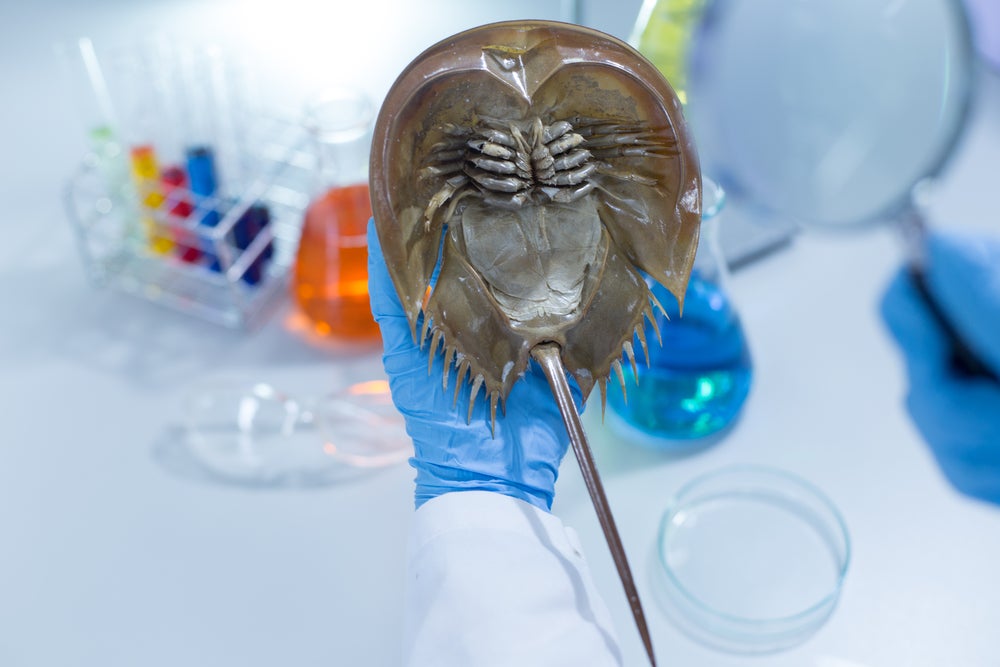
Horseshoe crabs are a species of marine arthropod that have existed for around 450 million years – but for over four decades, the creatures have also played a crucial role in human health.
The animals’ bright blue blood contains Limulus Amebocyte Lysate (LAL), a substance used by pharmaceutical companies the world over for bacterial endotoxin testing. LAL clots when it encounters bacterial endotoxins, enabling the detection of the potentially deadly contaminants in parenteral drugs, vaccines and medical devices.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Horseshoe crabs are the only natural source of LAL and are heavily relied upon by the pharmaceutical industry; over 500,000 are captured and bled every year. While LAL’s life-saving properties have made it a vital resource for drug and medical device companies, conservationists say the industry’s demand for horseshoe crabs is threatening their survival – and argue that with a synthetic alternative now available, bleeding the prehistoric creatures can no longer be justified.
Horseshoe bleeding: the ecological impact
Horseshoe crabs used for endotoxin testing in the US are harvested from the East Coast and drained of up to a third of their blood. Though the most of the animals are returned to the water after being bled, they don’t always survive; the mortality rate of horseshoe crabs released after bleeding has been estimated to be as high as 30%.
The number of horseshoe crabs spawned on the Delaware Bay, the prime harvesting spot for LAL companies, plummeted from an estimated 1.24 million in 1990 to just 333,553 in 2002. The local population appears to have remained at this level in the years since; a 2019 survey of the Delaware Bay counted 335,211 spawning horseshoe crabs. The American horseshoe crab is currently listed as vulnerable on the IUCN Red List, with biological resource use cited as a top threat to the future of the species.
It’s not just horseshoe crabs that suffer when they’re harvested on such a scale. A number of migratory birds, including the near-threatened red knot, rely on the calorie-rich horseshoe crab eggs laid onshore to fuel their 9000-mile journeys from South America to the Arctic. The decreased number of horseshoe crabs laying eggs on East Coast beaches poses a serious threat to the birds’ survival, as well as that of other animals for whom the eggs are a food source.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAn animal-free alternative
While LAL is considered the gold standard for endotoxin testing, a synthetic alternative to the blood-derived substance has been available for some time. In the 1990s, scientists at the National University of Singapore developed recombinant Factor C (rFC), a lab-synthesised, animal-free method of endotoxin testing. Rather than clotting around the pathogen like LAL does, rFC generates a fluorescent compound to indicate the presence of endotoxins.
The rFC assay has been commercially available since 2003, but uptake of the alternative by the pharma industry has been slow. Pharma giant Eli Lilly switched almost entirely to rFC back in 2016 – and has seen US approval for four rFC-tested therapies so far – but other companies are reluctant to follow suit.
Swiss multinational biotech Lonza, which also makes LAL, is one of only a small handful of rFC manufacturers worldwide. The company’s endotoxin testing solutions expert Allen Burgenson says pharma is hesitant to try new approaches when suitable, well-established options are already available.
“The pharmaceutical industry will always ask for bigger, better, faster, more – and then when you give it to them, they always say, ‘well, who else is using it?’” Burgenson says. “There’s a lot of aversion to risk; they don’t want to be the first, even though you’ve given them what they asked for.”
There is lively debate amongst scientists and companies about whether the synthetic product is comparable to traditional methods, but numerous studies have concluded that rFC is a suitable – and possibly even superior – alternative to testing with horseshoe crab lysate.
While Eli Lilly is the only company to publicly declare its use of rFC, Burgenson says several other pharma companies have begun to introduce the alternative into their testing processes.
“[They are] converting a lot of their testing to rFC, especially things like their water systems, where if it’s an upstream process material, there’s no requirement that you use an endotoxin detection reagent licensed by the Food and Drug Administration,” he explains.
Pharma’s stance on rFC
In 2012, the US Food and Drug Administration (FDA) published guidance including rFC as an acceptable alternative to traditional endotoxin testing – and, as seen in the approval of Eli Lilly’s rFC-tested products, the agency permits medicines to be tested in this way.
The US Pharmacopoeia (USP), however, is hesitant to embrace alternatives to LAL. The organisation, which sets quality standards for medicines, last year decided against making rFC endotoxin testing equal to the LAL-based method. The USP acknowledged the advantages of a synthetic alternative to LAL, but opted to put rFC guidelines in a stand-alone chapter, saying in a statement that there is currently insufficient evidence to demonstrate its equivalency.
This means US drugmakers are free to test their products with rFC, but must do extra validation work to prove their tests match those performed with crab lysate – no small factor in pharma companies’ reluctance to move away from traditional methods.
Across the pond, the industry is more welcoming of the animal-free alternative. In July 2020, the European Pharmacopoeia decided that rFC could be used for the bacterial endotoxin testing of substances and products, with no further validation required.
European Directorate of for the Quality of Medicines & Healthcare director Susanne Keitel said: “When used under appropriate conditions, rFC-based methods provide the same guarantee of a product’s compliance with the test for bacterial endotoxins – and therefore, of its safety for use in patients – as LAL-based methods.”
‘America’s being left behind’
Provided the two methods are equally effective, the benefits of adopting rFC over LAL are clear. Horseshoe crab-derived LAL is a finite resource, whereas rFC can be sustainably produced in unlimited quantities. The alternative removes the need to harvest a vulnerable species and synthetic products are more consistent when it comes to manufacturing and testing processes.
Ryan Phelan is co-founder and executive director of Revive & Restore, a California-based conservation non-profit. She says the leading US manufacturers of LAL – Lonza, Charles River Labs and Associates of Cape Cod – are standing in the way of American drugmakers’ adoption of rFC.
“You have three American companies with a vested interest in supporting an LAL, with an undue influence on the USP,” Phelan says. “We don’t have that influence on the European Pharmacopoeia, or the Japanese or Chinese, and so all of them are moving towards accepting it as the global standard.
“There are over 40 non-profit organisations involved in trying to lobby USP saying, ‘Enough already, you’ve got the data’,” she adds. “They keep requiring data of individual companies that makes it burdensome, and there are plenty of authoritative scientific publications that are saying this is just a stalling tactic.”
LAL maker Charles River has been outspoken about its hesitation to switch to rFC-based testing. According to the company, there isn’t enough data to show rFC is as reliable as LAL, and therefore using the alternative for endotoxin testing could pose a risk to patients.
In Phelan’s view, the US is “lagging way behind the rest of the world” in its reluctance to accept rFC as an alternative testing method, despite multiple studies demonstrating its equivalency.
“The gold standard has shifted to Europe,” she says. “America’s being left behind.”
Responsibility for animal welfare in the pharmaceutical industry is referred to as the three Rs: reducing the number of animals used, refining studies to minimise their impact on animals, and replacing animal experiments with alternatives wherever possible. In their continued reliance on LAL, Phelan says, drugmakers are failing to adhere to these principles.
“They have an obligation to their shareholders to actually start doing that, and nobody’s holding their feet to the fire,” she says. “It’s a little maddening because they can refine their production right now, using rFC in [their upstream water testing] with no challenge.”
For Burgenson, the industry’s adoption of rFC is “inevitable” – it’s just a question of when.
As far as Phelan is concerned, however, the world can’t afford to wait for US drug companies to decide to do the right thing; the time for the USP to catch up to other standard-setting organisations is now.
“We want to protect the horseshoe crabs for their own benefit,” she says. “They’ve been here for millions of years, and they belong on this planet.”




