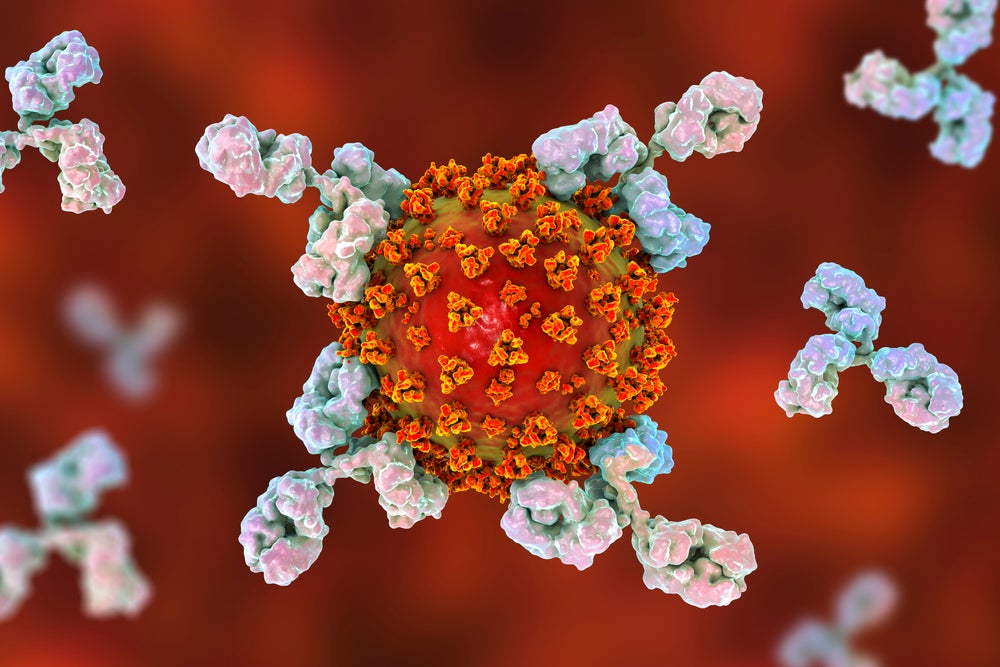
When a person is infected by a pathogen, such as a virus or bacteria, B cells (also known as B lymphocytes) recognise these foreign invaders and react by transforming themselves into plasma cells. These cells then produce Y-shaped proteins called antibodies that target the antigen found on the surface of that particular pathogen.
When antibodies bind to the antigens on the surface of a pathogen, they prevent it from entering other cells in the body and signal to other immune cells – known as phagocytic cells – to destroy that pathogen.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The next time a person is infected with that same pathogen, the process of producing antigen-specific antibodies by plasma B cell declines from a few weeks to just over a day. This part of the so-called adaptive immune response is known as immunologic memory and is a cornerstone to how the human body naturally fights infections and pathogens.
Covid-19 and antibodies
The Covid-19 pandemic has placed a spotlight on antibodies and their usefulness in medicine. There has been a lot of talk about antibody tests and their ability to determine if someone has been infected by SARS-CoV-2, the virus which causes Covid-19, and recovered.
Also, antibodies are at the heart of why vaccine research has been central to the pandemic response. Essentially, vaccines introduce a small, often inactivated piece of a pathogen into a patient’s body to stimulate this adaptive immune response, so if the person later contracts that disease, the immune system can respond much quicker.
A slightly less discussed use of antibodies in the pandemic is as therapeutics. Antibodies that have been found to neutralise SARS-Cov-2 virus may be a good way to treat Covid-19and prevent disease progression. Companies, including Eli Lilly and AstraZeneca, are currently studying neutralising antibody therapies in clinical trials.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataHowever, antibody-based drugs are not unique to Covid-19; they have been used for decades for various conditions, particularly autoimmune diseases and cancer. Experience of developing successful antibody drugs is what has enabled such quick scale-up of antibody discovery and clinical trials in the pandemic.
Let’s look back at the history of antibodies that has allowed these proteins to be used to fight the biggest threat to human health this century, Covid-19.
1890: First reference to antibodies in scientific literature
In 1890, German physiologist Emil von Behring and Japanese physician Kitasato Shibasaburo published an article about an animal experiment they had conducted in an attempt to treat diphtheria.
The researchers injected diphtheria into guinea pigs, then when they recovered from the disease, the scientists derived antitoxins (an early iteration of the term antibody) from the subjects’ blood plasma and injected them into other animals, including those with diphtheria symptoms. This protected these animals from developing diphtheria.
After these results were confirmed by further studies, Behring investigated the potential of using diphtheria antitoxins to treat humans of this deadly bacterial disease – this work lead to him winning the Nobel Prize for Medicine in 1901.
1890s – 1900s: Ehrlich makes further discoveries about antibodies
Paul Ehlrich, who worked alongside Behring on the diphtheria antitoxins research, wrote his own article in the early 1890s, in which he first used the term ‘antibody’. However, it took a few decades for this term to become routinely used in this immunological research.
Ehrlich also theorised how antibodies and antigens on pathogens interacted in a concept he called ‘selective theory’. It described how antibodies were specific to certain toxins (now known as antigens) and noted it was a lock and key interaction, which triggered the production of a heightened immune system.
This theory, which was disputed at the time, laid the groundwork for monoclonal antibody drugs used today in cancer treatment – Ehlrich’s theory was not properly proved until the 1940s.
Ehrlich was awarded the Nobel prize for Medicine in 1908 for his ground-breaking work on immunity.
During his research into cancer in the early 1910s, Ehrlich, with the help of his Japanese assistant, Sahachiro Hata, discovered arsphenamine, which combatted the bacteria that causes syphilis. After this discovery, Ehlrich began to look at ways to make chemotherapy specific – something which has continued to challenge the pharma industry. His theory – which he called a ‘magic bullet’ – aimed to find a way to deliver chemotherapy with a selective agent, such as an antibody.
1975: Invention of mAbs
While working at the Medical Research Council (MRC) in Cambridge, Argentine César Milstein and German Georges Köhler developed the first monoclonal antibodies (mAbs) –antibodies made by immune cells that are clones of a parent cells and bind to one particular antigen. Importantly, they manged to do it at scale that enhanced mAbs’ potential for clinical use.
To do this, they developed a hybridoma technology that fused cells from an animal myeloma cell line with spleen cells from a mouse immunised with sheep red blood cells.
They then incubated the cells for two weeks and used the hybridoma to secrete large quantities of mAbs specific for the sheep red blood cells. Milstein and Köhler received the Nobel Prize for Medicine in 1984 for this discovery.
1986: Approval of first mAb, Orthoclone OKTE
It took a decade after Köhler and Milstein’s discovery at the MRC for a safe and effective mAb-based drug could be developed. The first approved mAb was Janssen’s Orthoclone OKT3 (muromonab-CD3) and it was indicated for preventing kidney transplant rejection. It was developed using an improved variation of Milstein and Köhler’s hybridoma technology.
Orthoclone OKT3 is a monoclonal IgG2a antibody targeting the CD3 antigen, which is on the surface of another immune cell, T cells. The aim of the mAb is to prevent T cells attacking the transplant, which they deem to be foreign objects.
1990s: The need to humanise mAbs
Over the next decade, scientists continued to experiment with different techniques to try and further modify mAbs to reduce the immune response against the therapy itself. Improvements would make it possible to develop therapies for conditions that need long-term treatment, such as cancer.
This led to the approval of daclizumab for preventing the rejection of kidney transplants – the drug was later approved for multiple sclerosis. Researchers at Protein Design Labs humanised a mouse mAb called anti-Tac by modifying the xenogeneic antibody sequences to include more human sequences than mouse ones. This technique is called complementarity determining regions (CDR) grafting.
The 1990s and 2000s saw significant growth in the number of humanised mAbs being developed and approved– examples include Genentech’s Herceptin for Crohn’s disease, Biogen’s Rituxan for non-Hodgkin lymphoma and AbbVie’s Humira for rheumatoid arthritis. The latter has been the world’s best-selling prescription drug for several years.
The techniques used to humanise mAbs have improved from CDR grafting to phage display technologies, which was invented by George Smith in the 1990s and used to develop Humira, as well as transgenic mice.
mAbs have continued to be predominant in modern medicine – as of December 2019, there are 79 approved mAbs; 30 of these are for cancer. The global therapeutic market for mAbs was valuated at $115.2bn in 2018; this is expected to increase to $300bn by 2025.
2000s – 2010s: ADCs enter the market
Building on Ehlrich’s vision of using antibodies to introduce chemotherapy selectively to cancer cells, almost a century later, researchers developed a concept called an antibody-drug conjugate (ADC).
The first ADC approved was Pfizer’s Mylotarg (Gemtuzumab ozogamicin) for acute myeloid leukaemia in 2001. It combines mAb gemtuzumab with chemotherapy ozogamicin to selectively target the CD33, which is found on the surface of some leukaemia cells.
Unfortunately, Mylotarg was not particularly safe in some patients, so it was withdrawn from the market in 2010. In other ADCs developed in the 2000s, issues arose around the stability and low dose load capacity of chemical linkers used to connect the two therapeutic agents.
However, in the last decade, significant progress has been made in terms of improving the safety and efficacy of ADCs, particularly in regard to the linker. One recent approval is Immunomedics’ Trodelvy (sacituzumab govitecan) for metastatic triple negative breast cancer – this ADC relies on a different type of linker, a hydrolysable linker that allows a high chemotherapy payload to be attached to the mAb, improving efficacy of the drug.
2009: Towards bispecific Abs
mAbs are monospecific molecules that bind to one specific antigen on the pathogen, meaning they are sometimes unable to treat tumours as a monotherapy. Consequently, in the 1960s, researchers began looking at the bispecific Abs (BsAbs). These therapies are engineered to allow a single antibody to bind to two different antigens, and as such they can better recruit other elements of the immune system, such as T cells, to also target pathogenic ells.
It took until 2009 for the first BsAb to gain regulatory approval Europe. Developed by Trion Pharma, Catumaxomab targets both CD3 and EpCAM to treat solid tumours – it was removed from the market for commercial reasons in 2017. Since then, two BsAbs have been approved – blinatumomab for acute lymphoblastic leukemia in 2014 and emicizumab for haemophilia A in 2017.
A few companies have begun to look further towards multi-specific antibody drugs for cancers. One example is Numab, which has developed a technology platform that aims to overcome the limits of mAbs by creating an antibody drug with a mechanism of action that would not be possible by combining multiple different separate agents.
2020: Covid-19 and neutralising antibodies
Early into the pandemic, antibody discovery companies and researchers began to look for antibodies that selectively bound to the SARS-CoV-2 virus and inhibited its capacity to infect healthy human cells. The idea was that these antibodies could be turned into mAbs to neutralise the virus to both prevent disease progression and treat patients infected in the patients.
There is also hope that these neutralising antibody drugs could be used to prevent Covid-19 in patients who have not yet contracted the virus – they would be particularly useful for patients who are unable to receive a Covid-19 vaccinedue to pre-existing conditions.
AstraZeneca is working on combining two mAb drugs that bind to different parts of the SARS-CoV-2 virus’ spike protein, which is the part of the virus responsible for binding and infecting human cells.
The company, which is collaborating with Vanderbilt University on this project, plans to start two clinical trials of these drugs later this year. AstraZeneca has already signed a supply deal with the UK Government’s Vaccine Taskforce for one million doses of these mAbs.
Slightly further ahead is Eli Lilly, which partnered with Canadian-based AbCellera, on developing a mAb called LY-CoV555. It is a neutralising IgG1 mAb directed against the spike protein of the SARS-CoV-2 virus. This drug entered Phase III trials at the beginning of August with the support of the US National Institute of Allergy and Infectious Diseases.
Is digitally printed packaging now the best option for CPG companies? Has the Covid-19 pandemic accelerated the adoption of digital printing by consumer brand owners? Our short survey aims to find out. View the survey here and complete to be among the first to see the results.




