
For researchers working in the field of public health and addiction, long-term opiate users pose a conundrum. While most users do respond to conventional opioid substitution therapies such as methadone, a small subset do not benefit and remain dependent on street drugs.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
For these users, medically-supervised diamorphine injections (the generic name for pharmaceutical grade heroin) may lead to the best outcomes, eradicating many of the associated social and personal harms. And while the idea may sound counterintuitive – why would you treat heroin addiction with the very drug that’s causing the problem? – the evidence points clearly in that direction.
Trial phase
In 1994, Switzerland conducted the first large-scale trials into heroin-assisted treatment. The findings were unequivocally positive: users were at lower risk of overdose and blood-borne diseases, saw improvements in health and social stabilisation, and were less likely to turn to crime. Subsequent trials – in the Netherlands, Germany, Spain, Canada and the UK – have proven similarly encouraging.
In the UK, where specially-licensed doctors have been allowed to prescribe diamorphine since as early as 1926, there has been a push to make heroin maintenance programmes a legitimate part of the NHS. In several other European countries, such as Germany and the Netherlands, the treatment is already part of routine procedure. While it is never a first port of call, it has been a lifeline in cases where nothing else has worked.
The difficulty arises in countries where that isn’t an option. With the United Nation’s ‘war on drugs’ still going strong, many healthcare systems must operate within prohibitionist constraints, making diamorphine illegal to prescribe. This creates a gap in services for the most vulnerable users.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
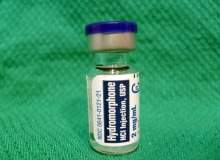
By GlobalDataLuckily, this could be set to change, following the publication of groundbreaking research in the Journal of the American Medical Association (JAMA) Psychiatry in April. The SALOME project found that a common pain medication – hydromorphone – might be just as effective as pharmaceutical-grade heroin for users who hadn’t responded to other treatments.
"We don’t have pharmaceutical-grade heroin licensed here in Canada – it’s not a treatment we can offer," explains principal investigator Dr Eugenia Oviedo-Joekes, associate professor in the University of British Columbia’s (UBC) School of Population and Public Health. "So we tried hydromorphone, which is a licensed drug in most countries because it’s used for pain relief. We showed that this drug is as good as pharmaceutical-grade heroin, giving us an opportunity to expand this supervised model of care and treat the people who need it."
Safety first
SALOME, which stands for the Study to Assess Longer-term Opioid Medication Effectiveness, was led by researchers from Providence Health Care, St Paul’s Hospital and UBC. Running from 2011 until 2015, this was the first trial of its kind in the world.
It followed on from the North American Opiate Medication Initiative (NAOMI) in 2009, which had found that "prescribed, supervised use of diacetylmorphine [a synonym for diamorphine] appears to be a safe and effective adjunctive treatment for [the] severely affected population of patients who would otherwise remain outside the healthcare system." Unfortunately, the researchers did not receive federal approval to continue this intervention.
The SALOME study aimed to find another way. It recruited 202 participants in Vancouver, all of whom had at least five years of documented drug addiction and who had been using heroin frequently for at least a year. Each participant was randomly assigned to receive injectable diamorphine or hydromorphone, up to three times daily, for six months under medical supervision. Because this was a double blinded trial, neither the patients nor the doctors treating them knew which drug they were receiving.
At the end of the study period, both groups of users had seen benefits – all reported fewer days of street heroin use, averaging three to five days per month of illicit drug use, compared to almost daily injections beforehand. Crime rates had plummeted too, with participants reporting less than four days of illegal activities per month, down from 14.1.
Importantly, both drugs proved safe when taken in a clinical setting. Out of a total of 88,451 injections, there were 29 serious adverse events (including 14 overdoses and 11 seizures), which might have proven fatal in a street setting. Only five of these occurred in the hydromorphone group, suggesting this drug might actually have the edge in terms of safety.
"When you prescribe this drug under supervision, it’s safe because if the patient overdoses they can take a medication to reverse it," says Oviedo-Joekes. "This is an important message – under supervision nothing happens because there are nurses there to treat you, and there were only 14 overdoses that required treatment out of 88,000 injections."
The results presented strong grounds for suggesting hydromorphone is ‘non-inferior’ to diamorphine. At the end of the trial, the participants were not able to reliably guess which drug they had been taking, which is nothing short of remarkable in a population so accustomed to heroin.
Politically palatable
Of course, the benefits of the intervention went beyond supervised usage. The participants were exposed to an interdisciplinary team of doctors, nurses, social workers and counselors, who worked with them to help them find stability in their lives (including looking for jobs and housing). They were also given primary care services, psychiatric help and medications for conditions like HIV. As Oviedo-Joekes explains, one of the key benefits of heroin-assisted treatment is that it brings users back into the healthcare system.
"So far, the Netherlands, Denmark, Switzerland and Germany are doing very well with a supervised model of care," she says. "They’re all taking very different approaches, but what those countries have in common is that they say to users – you come here, we provide you with the medication and this ensures your safety and the safety of the community but also gives us an opportunity to provide comprehensive care. This model of care is about the people – how can I have you close enough to help you?"
Oviedo-Joekes is hopeful that hydromorphone might prove more politically palatable than medical heroin, giving hope to chronic users in countries where diamorphine is not available.
"Locally, we’re now trying to build a network where we can expand these clinics and offer hydromorphone as another alternative for supervised injection," she says. "Internationally we hope that people that need help can start with the hydromorphone while they wait for diamorphine to be licensed."
Addiction research, she points out, differs from other areas of medicine in an important capacity – the barriers to implementing new treatments are not just financial but political. Advocacy work is therefore essential.
"Opiate dependency requires an open-minded way of treating, and we need to treat out patients in the same way we’d treat patients with diabetes or a heart condition," she says. "Having more treatments available benefits everybody, so I hope this evidence helps policymakers move forward. These supervised models of care are just a small part of the addiction system and they’re not meant to replace anything – it’s just another way of caring for the most vulnerable."




