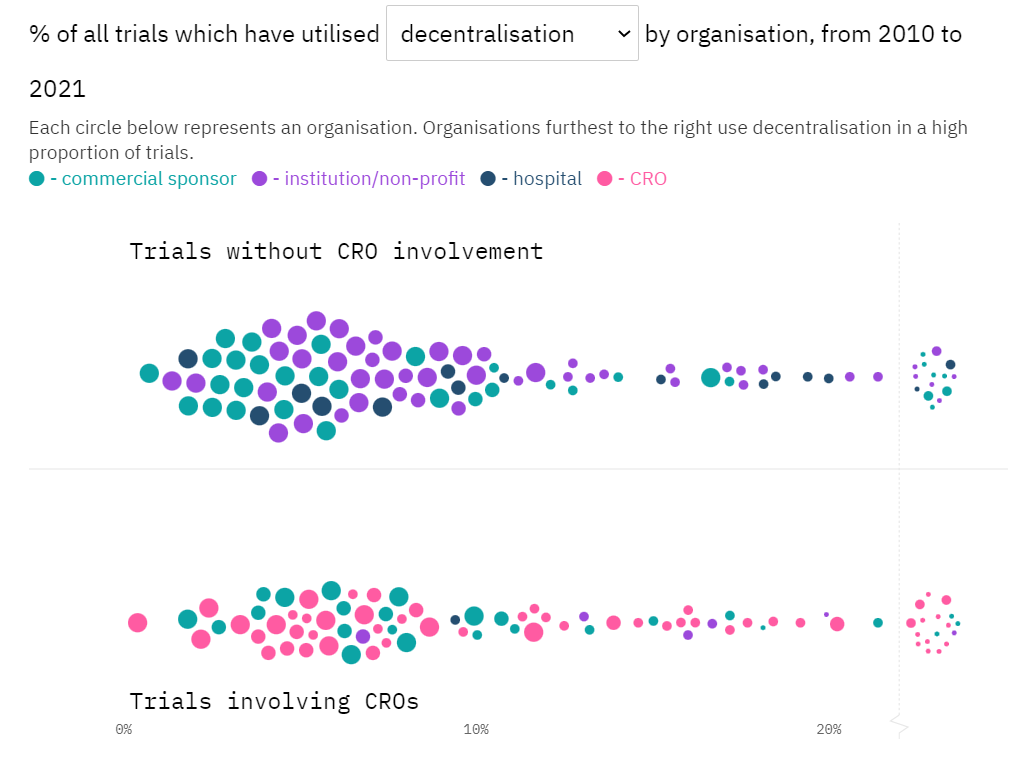
Much has been said about the boom of decentralised clinical trials (DCTs) over the past couple of years, but not enough has been done to characterise the series of seemingly analogous elements and approaches that can make a clinical trial decentralised.
The abundance of trial activities that can be conducted remotely or supported by digital tools has given rise to a nomenclature that is equivocal. And this has fuelled an ambiguous collective conversation in which “decentralised” trials are not being perceptibly different from each other, even if in practice they can be quite distinct in their approach and impact.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
While some of these remote or virtual approaches have been around for some time, we may be witnessing a true paradigm shift in the way clinical research is conceived. At Clinical Trials Arena we feel it is time to get past the understandable hype-talking stage (these approaches have kept trials running during very challenging times) and help the clinical trial ecosystem ride this tidal adoption wave.
Through a pioneering taxonomic lens powered by GlobalData, we are introducing the first analysis of a series based on a global DCT adoption tracker. These analyses will provide key insights on the adoption trends of specific decentralisation approaches and components, whether digital or otherwise. These valuable insights will help you, our audience, understand the DCT landscape by therapeutic area, geography, sponsor, CRO involvement, and other relevant trial characteristics.
Our goal is to facilitate the characterisation of DCT archetypes across the evolving trial decentralisation continuum while we report on one of the most fascinating developments in the clinical research field to date. As evidence for impact on clinical development KPIs starts to mount, and a plethora of decentralisation approaches continue to flow into sponsor and CRO toolkits, every month we will deploy our best data-led reporting to deliver the latest on the clinical trial decentralisation landscape.
The Clinical Trials Arena editorial team is committed to contributing to the collective understanding of how DCTs are meeting patients where works best for them and for the effective generation of clinical evidence.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataPharmaceutical Technology, Hospital Management, Medical Device Network, and Clinical Trials Arena are all part of GlobalData Media Pharma & Healthcare.
Read more on Clinical Trials Arena:
DCT Adoption Tracker: Who and what is at the crest of the trial decentralisation wave?
In an exclusive analysis, Clinical Trials Arena combed through drug trial public information to find out which decentralised approaches are making waves and talked to experts to put trends into context. Read more…
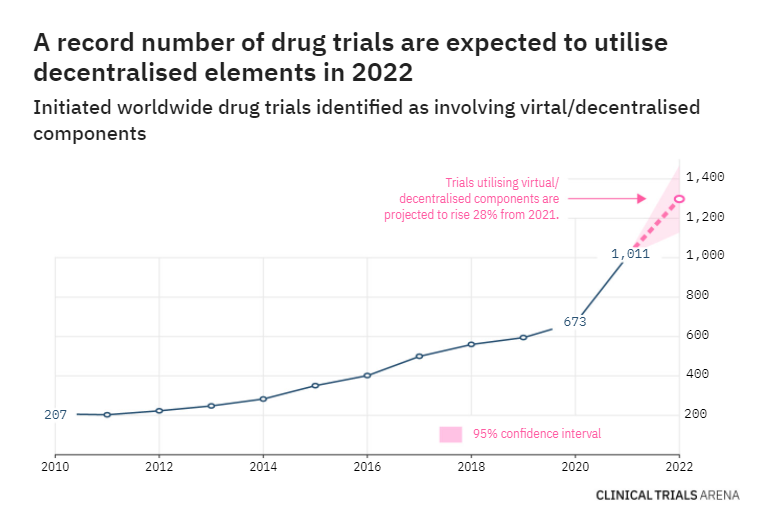
2022 forecast: decentralised trials to reach new heights with 28% jump
About 1,300 drug clinical trials with a virtual and/or decentralised component are forecasted to start in 2022, representing a 28% increase from 2021. For details, click here.