Pharmaceutical and biotech companies live and die by regulatory decisions on their therapies. These verdicts not only impact the company that developed the particular therapy, but also those in charge of manufacturing it.
In this ongoing series, Pharmaceutical Technology takes alook at recent regulatory rulings that will likely impact the manufacturing volumes of drugs and biologics. Through this, we shine a light on the role of contract manufacturing organizations (CMOs), an important stakeholder in the pharma landscape, and their relationships with pharma companies.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
This analysis covers the period of late mid-June to late July and is based on a list of CMOs that will likely be impacted by regulatory decisions by the US Food and Drug Administration (FDA), European Medicines Agency (EMA), and reimbursement authorities like the UK’s National Institute of Health and Care Excellence (NICE).
These outsourcing contracts involve parenteral manufacturing, packaging, biologic active pharmaceutical ingredient (API) manufacturing, and more. This analysis is based on the GlobalData Pharma Intelligence Center’s Deals database and PharmSource reports.
Source: GlobalData Pharmaceutical Intelligence Center
Covid-19 vaccines continue to advance
In the last few months, Valneva has seen its share of mixed regulatory decisions for its Covid-19 vaccine VLA2001. The company had a EUR 1.37 billion supply deal with the UK government, and a manufacturing facility was going to be set up in Livingston, West Lothian, Scotland. But in late 2021 the government terminated the deal. In April, the EMA requested additional data from the company, and the European Commission (EC) also announced it planned to terminate an advance purchase agreement if the vaccine was not authorized by the EMA before April 30. On June 23, however, the vaccine did receive a marketing authorization, and last month, the EC approved an amended purchase deal for the vaccine. IDT Biologika has been charged with manufacturing the biologic API for it.
Novavax’s Covid-19 vaccine has been in development for a relatively long time as well, before receiving recent positive verdicts. On July 6, the EC approved the expansion of its conditional marketing authorization to include adolescents of ages 12–17 years. This was soon followed by an FDA emergency use authorization (EUA) for Nuvaxovid’s use in adults. The vaccine’s biologic API manufacturing has been outsourced to Biofabri, Fujifilm Diosynth Biotechnologies USA, Mabion, SK Bioscience, and Emergent Biosolutions. In addition, the parenteral manufacturing is contracted to Baxter Biopharma and Emergent.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataCardiovascular and obesity drugs hit their stride
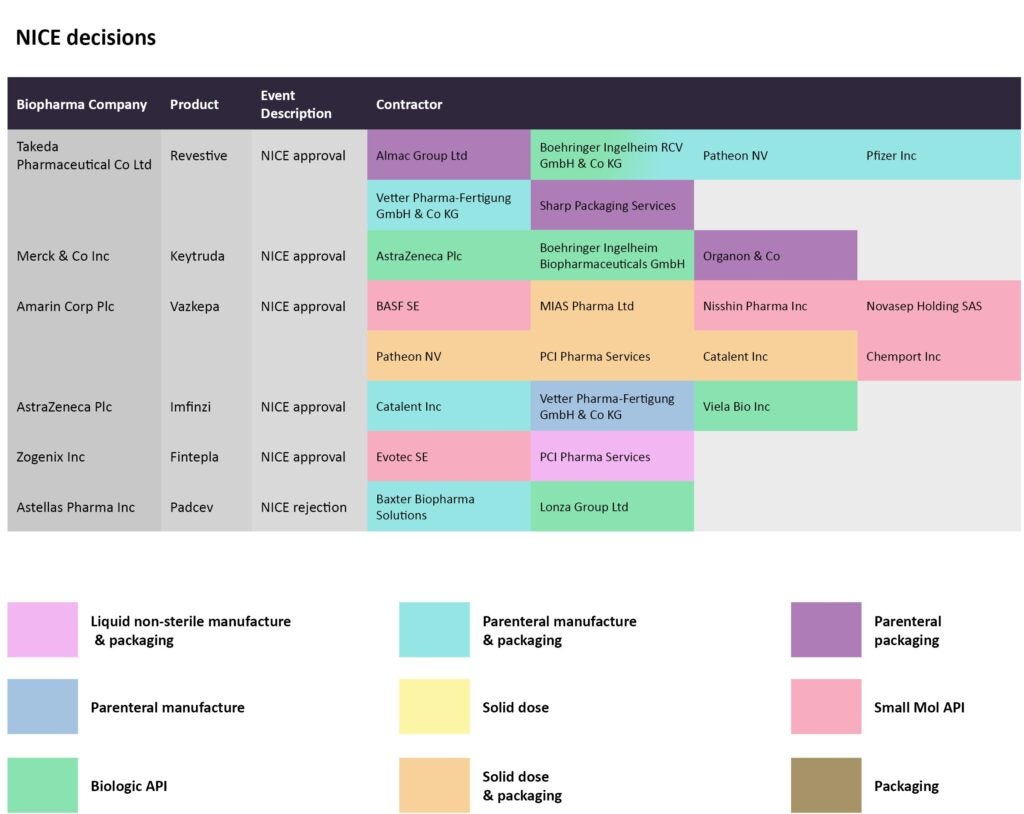
In mid-June, NICE recommended the use of Amarin’s Vazkepa to prevent cardiovascular events like heart attacks and strokes. The small molecule’s manufacturing has been outsourced to several CMOs; BASF SE, Nisshin Pharma, Chemport, and Novasep are producing the small molecule API, while MIA Pharma, Patheon by ThermoFisher Scientific, and PCI Pharma Services are taking charge of solid dose and packaging.

Source: GlobalData Pharmaceutical Intelligence Center
In the metabolic disorders space, an expanded label from the FDA now means that Rhythm Pharmaceuticals’ Imcivree can be prescribed for chronic weight management in adult and pediatric patients six years of age and older with obesity due to Bardet-Biedl syndrome (BBS). Corden Pharma International, Polypeptide Group, and Recipharmare contracted to participate in different stages of its manufacturing.
The label for Horizon Therapeutics’ Krystexxa was expanded to include its use as a treatment, given with methotrexate, for patients with uncontrolled gout. Bio-Technology General Israel, Fujifilm Diosynth, and NOF Corp are responsible for manufacturing the biologic API for the recombinant uricase therapy. In the oncology arena, checkpoint inhibitors by Merck & Co and AstraZeneca received NICE approvals for use in combination with chemotherapy for metastatic triple-negative breast cancer, and as a maintenance therapy for treating non-small cell lung cancer after platinum-based chemoradiation, respectively. With the former, Organon has been contracted for parenteral packaging for Keytruda, while the parenteral manufacturing for the latter’s Imfinzi has been outsourced to Vetter Pharma-Fertugung.
To read the previous edition of this series, click here and here.





