Zydus Lifesciences has filed a patent for a therapeutic compound to prevent and treat primary biliary cholangitis (PBC). The compound, saroglitazar magnesium salt, is administered orally once daily to effectively treat PBC. GlobalData’s report on Zydus Lifesciences gives a 360-degree view of the company including its patenting strategy. Buy the report here.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
According to GlobalData’s company profile on Zydus Lifesciences, NSAID cancer drugs was a key innovation area identified from patents. Zydus Lifesciences's grant share as of June 2023 was 1%. Grant share is based on the ratio of number of grants to total number of patents.
Treatment for primary biliary cholangitis using saroglitazar magnesium salt
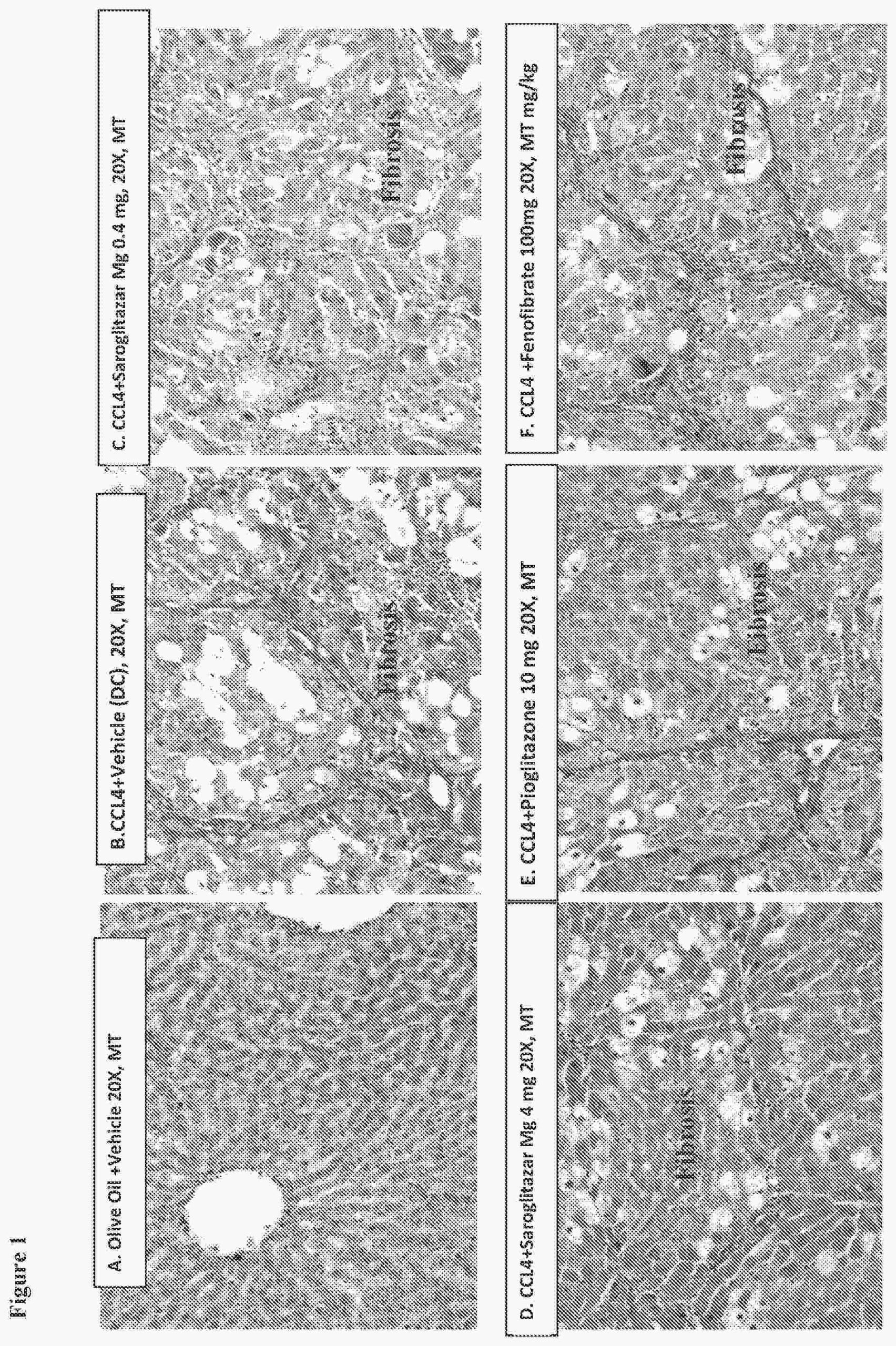
A recently filed patent (Publication Number: US20230201162A1) describes a method for treating primary biliary cholangitis, a liver disease, using a specific compound called saroglitazar magnesium salt. The method involves administering this compound orally and once daily to a human in need of treatment. The patent claims that saroglitazar magnesium salt is the sole therapeutic agent for treating primary biliary cholangitis.
According to the patent claims, the method can be carried out using different doses of saroglitazar magnesium salt. Claim 2 states that about 4 mg of the compound is administered, while claim 3 mentions a lower dose of about 2 mg. The administration of saroglitazar magnesium salt is intended to occur for at least 16 weeks, as stated in claim 5.
In addition to the method of administration, the patent also describes a pharmaceutical formulation of saroglitazar magnesium salt in the form of a tablet or a capsule. This formulation includes the compound along with a pharmaceutical excipient. Claim 7 specifies that saroglitazar magnesium salt is the sole therapeutic agent in this pharmaceutical formulation.
The patent claims provide options for the dosage of saroglitazar magnesium salt in the pharmaceutical formulation, with claim 9 stating about 2 mg and claim 10 mentioning about 4 mg.
Overall, this patent outlines a method for treating primary biliary cholangitis using saroglitazar magnesium salt as the main therapeutic agent. The method involves oral administration of the compound once daily for at least 16 weeks. The patent also describes a pharmaceutical formulation of saroglitazar magnesium salt in the form of a tablet or a capsule. The dosage of the compound can vary between about 2 mg and about 4 mg in both the method of administration and the pharmaceutical formulation.
To know more about GlobalData’s detailed insights on Zydus Lifesciences, buy the report here.
Data Insights
From

The gold standard of business intelligence.
Blending expert knowledge with cutting-edge technology, GlobalData’s unrivalled proprietary data will enable you to decode what’s happening in your market. You can make better informed decisions and gain a future-proof advantage over your competitors.



