The pharmaceutical industry continues to be a hotbed of innovation, with activity driven by the evolution of new treatment paradigms, and the gravity of unmet needs, as well as the growing importance of technologies such as pharmacogenomics, digital therapeutics, and artificial intelligence. In the last three years alone, there have been over 633,000 patents filed and granted in the pharmaceutical industry, according to GlobalData’s report on Innovation in Pharmaceuticals: Tetrapeptide derivatives. Buy the report here.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
However, not all innovations are equal and nor do they follow a constant upward trend. Instead, their evolution takes the form of an S-shaped curve that reflects their typical lifecycle from early emergence to accelerating adoption, before finally stabilising and reaching maturity.
Identifying where a particular innovation is on this journey, especially those that are in the emerging and accelerating stages, is essential for understanding their current level of adoption and the likely future trajectory and impact they will have.
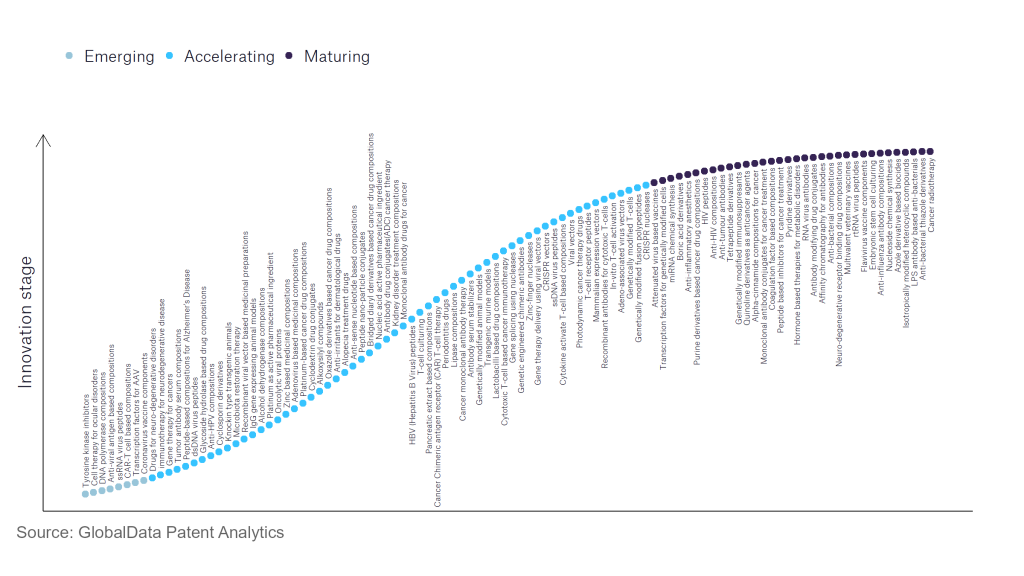
110 innovations will shape the pharmaceutical industry
According to GlobalData’s Technology Foresights, which plots the S-curve for the pharmaceutical industry using innovation intensity models built on over 756,000 patents, there are 110 innovation areas that will shape the future of the industry.
Within the emerging innovation stage, cell therapy for ocular disorders, coronavirus vaccine components, and DNA polymerase compositions are disruptive technologies that are in the early stages of application and should be tracked closely. Adeno-associated virus vectors, alcohol dehydrogenase compositions, and antibody serum stabilisers are some of the accelerating innovation areas, where adoption has been steadily increasing. Among maturing innovation areas are anti-influenza antibody compositions and anti-interleukin-1, which are now well established in the industry.
Innovation S-curve for the pharmaceutical industry
Tetrapeptide derivatives are a key innovation area in the pharmaceutical industry
Tetrapeptides are classified as oligopeptides as they contain only four amino acids linked by peptide bonds. Several tetrapeptides are pharmacologically active, showing affinity and specificity for multiple receptors in protein-protein signalling. In nature, tetrapeptides (CTPs) are both linear and cyclic, with the latter mimicking reverse turns commonly found on the surfaces of proteins and targets for drug therapy. The tetrapeptide derivatives L-lysine-L-arginine-L-aspartic acid-L-serine (tetrapeptide A) and di-tert.butyloxycarbonyl-L-lysine-L-arginine-L-aspartic acid-tert.butyl O-tert.butyl-L-serinate (tetrapeptide B) have demonstrated optimal cardioprotective effect. Tetrapeptide derivative PEP1261 (BOC-Lys(BOC)-Arg-Asp-Ser(tBu)-OtBU), a peptide sequence (39-42) of lactoferrin inhibited the effect on the ROS generation as well as in mRNA synthesis and expression of complement factors in neutrophils isolated from infarct heart.
GlobalData’s analysis also uncovers the companies at the forefront of each innovation area and assesses the potential reach and impact of their patenting activity across different applications and geographies. According to GlobalData, there are 170+ companies, spanning technology vendors, established pharmaceutical companies, and up-and-coming start-ups engaged in the development and application of tetrapeptide derivatives.
Key players in the tetrapeptide derivatives space – a notable innovation area in the pharmaceutical industry
‘Application diversity’ measures the number of different applications identified for each relevant patent and broadly splits companies into either ‘niche’ or ‘diversified’ innovators.
‘Geographic reach’ refers to the number of different countries each relevant patent is registered in and reflects the breadth of geographic application intended, ranging from ‘global’ to ‘local’.
Amgen is the leading patent filer of tetrapeptide derivatives. Amgen develops, manufactures, and markets innovative human medicines to treat patients suffering from serious diseases. It develops novel medicines in six focused disease areas including cardiovascular diseases, oncology/haematology, inflammation, bone health, neurological disorders and nephrology. Carfilzomib (Kyprolis), an approved treatment for multiple myeloma, is a synthetic tetrapeptide developed by Amgen. Amgen is headquartered in Thousand Oaks, California, US. Other players in the space include Bristol-Myers Squibb and F. Hoffmann-La Roche with key patent filed by both companies for tetrapeptide derivatives as potential drug targets.
In terms of application diversity, Aeterna Zentaris is the top company, followed by Dr. Wolff Gruppe and A. Menarini Industrie Farmaceutiche Riunite. By means of geographic reach, Curis holds the top position. Whilst Abliva and Ambrx Biopharma stand in second and third positions, respectively.
To further understand the key themes and technologies disrupting the pharmaceutical industry, access GlobalData’s latest thematic research report on Pharmaceutical.
Data Insights
From

The gold standard of business intelligence.
Blending expert knowledge with cutting-edge technology, GlobalData’s unrivalled proprietary data will enable you to decode what’s happening in your market. You can make better informed decisions and gain a future-proof advantage over your competitors.



