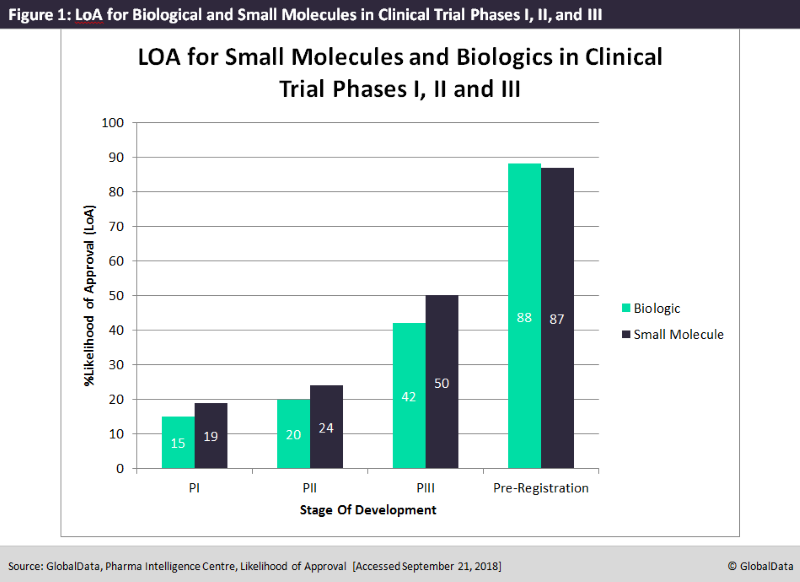
According to GlobalData’s new Likelihood of Approval (LoA) tool, small molecules are 22% more likely to be approved than biologics when comparing LoA for Phase I, II, and III clinical trials combined. GlobalData’s LoA tool allows clients to determine the probability of a drug with specific characteristics progressing to the next stage of clinical development, as well as the ultimate probability of the drug receiving market authorization based upon its historical data.
Figure 1 below was generated using this tool, and shows that small molecules have a distinct lead over biologics in LOA at each stage of clinical development. In Phase I of clinical trials, small molecules have a 27% greater LoA than biologics, but this decreases to only 20% in Phase II and to 19% in Phase III. However, once past clinical stages and into pre-registration, small molecules display no advantages in LOA over biologics and in fact exhibit nearly the same percentage likelihood of receiving market approval.
Despite this predominance in LoA for small molecules during Phases I, II, and III, biologics are continually growing; this trend is evident in big pharmaceutical companies such as Bayer, which is aiming to expand its biologic molecules offering to account for one-third of its entire pipeline.


US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData