On 28 June, the United States Department of Justice announced numerous healthcare fraud enforcement actions targeting schemes billing payers for medically unnecessary compounded drugs, including compounded pain and scar creams.
Compounding pharmacies allegedly included specific combinations of ingredients in compounded creams to increase the billing amount, such as active ingredients in drugs approved by the FDA for non-topical administration to treat non-pain related indications, such as antidepressants, anticonvulsants, antivirals, and narcotics. The relative lack of information surrounding these compounded topical creams means that possible weak efficacy and safety risks may go unnoticed by the medical community and reimbursement schemes. The FDA is therefore planning to increase and enhance the information available on compounded topical pain creams.
Compounding is a practice in which an authorised person (normally a pharmacist or physician) combines, mixes, or alters ingredients of a drug to create a medication tailored to the needs of an individual patient. It is used to alter dosage strength, dosage forms and flavour, and to exclude certain ingredients that the patient has an allergy or intolerance to. It allows for a more patient-specific pharma product, whereas medicines manufactured by large companies are normally a one-size-fits-all approach, which causes problems for certain patients, particularly those in the paediatric and geriatric populations.
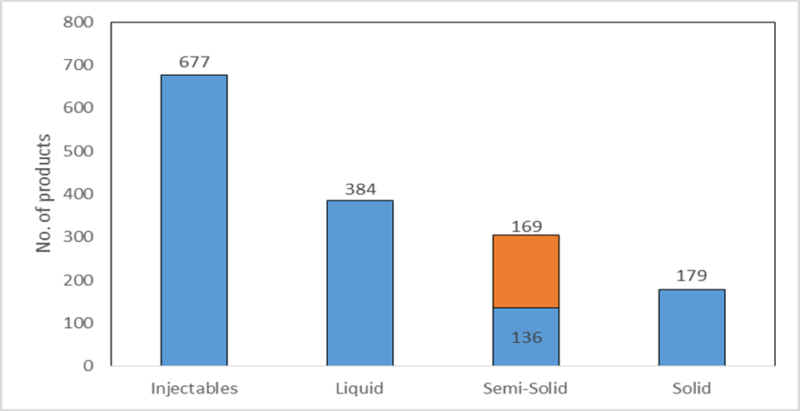
The following figure shows the dosage forms of products the registered 503B facilities were compounding between December 2016 and May 2017. The FDA has designated 503B compounding pharmacies as those with outsourcing facilities that may manufacture large batches with or without prescriptions to be sold to healthcare facilities for office use only. The largest dosage form group is injectables (44% of all compounded products), which are required to be sterile; this indicates that the most commonly found violation by compounding pharmacies, contaminated products, is particularly serious. Of the semisolids, creams (the segment in orange) form the majority, with 48 of the 169 creams containing an analgesic as an active ingredient – a substantial number of products that may have been affected by the issue discovered.
Figure 1: dosage form of products compounded by 503B facilities


US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataSource: FDA, 2017. © GlobalData
Although compounded drugs are administered to patients in the US, they are not technically FDA approved. The FDA has recently introduced further measures to promote the use of approved drugs and minimise the risks to patients who are prescribed a compounded drug. These include:
- Assessing if there is a clinical need for compounding drugs from bulk drug substances;
- Inspecting compounding facilities to assess if drugs that are essentially copies of FDA-approved drugs are being compounded for patients who could use an FDA-approved drug;
- Inspecting compounding facilities not registered as outsourcing facilities to confirm that they are distributing compounded drugs according to valid patient-specific prescriptions.
In the last six years, compounding has received negative press due to the 2012 meningitis outbreak involving the New England Compounding Center, resulting in 793 becoming ill and 64 deaths. Since the event, the regulated environment for US compounding pharmacies has become far more stringent. The Drug Quality and Security Act (DQSA) was enacted by Congress on November 27, 2013, resulting in large-scale compounders having to register as outsourcing facilities with the FDA and becoming subject to Current Good Manufacturing Practice compliance and FDA inspection.
Research by PharmSource, a GlobalData product, shows that 15% of all FDA-registered compounding facilities in the US received Warning Letters between 2014–2017, recalled products, or have been closed by the FDA based on their last FDA inspection. It is startling that such a large proportion has not complied with regulations. Of the seven Warning Letters issued since last inspection (most occurred between 2016-2018 for 65 facilities), only five were publicly viewable. Adulterated or contaminated drug products were the most common violation. This was followed by three misbranded drug product violations, where labeling failed to provide adequate directions for their intended uses by non-medical practitioners. Warning Letters were issued in two instances for failure to report drugs being compounded and unapproved new drug products being used for compounding.
Figure 2: number of violations or actions against non-compliant 503B facilities since last inspection

Source: FDA, 2017. © GlobalData
Compounding pharmacies play an important role during drug shortages, providing discontinued drugs to patients and supplying drugs to hospitals. When there is no approved or even off-label treatment for rare diseases, pharmacists can also compound drugs to produce a specific treatment for patients (Doom and Carvalho, 2018). Before the age of pharmaceutical mass manufacture, almost all prescriptions were compounded by pharmacists. PharmSource predicts that this model will become increasingly common once again as demand for personalised medicines rises, despite US regulation seeking to curb this activity. There is certainly a place for compounding, but it must be subject to stringent regulations if it is to properly benefit the treatment landscape, avoid scandals, and – most importantly – not jeopardise patient safety.
Industry updates such as this are covered in the Bio/Pharma Outsourcing Reports from PharmSource, a GlobalData product. If you do not subscribe to PharmSource or the Bio/Pharmaceutical Outsourcing Report, please contact a GlobalData sales representative to gain access.