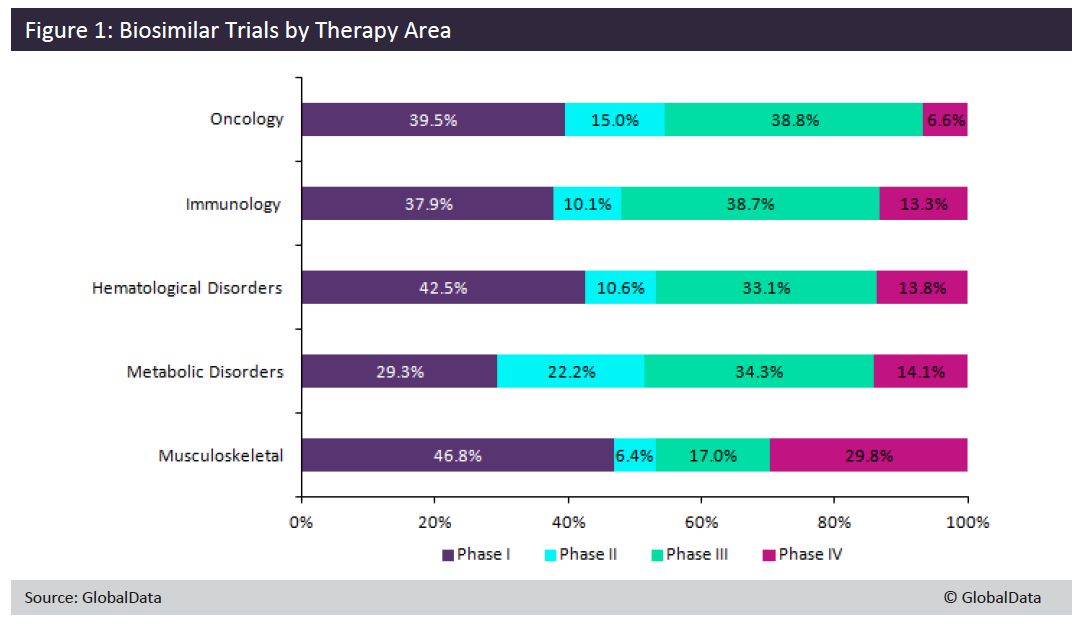
Over the past decade, the number of clinical trials using a biosimilar treatment has been on the rise. GlobalData extracted all biosimilar drug trials up to December 21, 2018 and then broke them down by therapy area.
The largest number of biosimilar clinical trials took place in oncology, followed by immunology, hematological disorders, metabolic disorders, and musculoskeletal.

When split into phases GlobalData found biosimilar trials were most numerous in Phase I and Phase III across the major therapy areas (Figure 1). Musculoskeletal had the largest number of Phase I trials and a much higher number of Phase IV trials than any other therapy area. One of the largest treatment areas for approved biologic drugs is arthritis, so this may explain why there are so many post-marketing trials in this therapy area.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData