Huntington disease is an inherited autosomal dominant neurological disorder that leads to the progressive degeneration of the brain’s nerve cells. In Huntington disease, the abnormal gene causes an excess of chromosomal trinucleotide repeats on chromosome 4p16.3, leading to the production of a toxic protein called huntingtin.
Despite significant research efforts made toward treating the disease, no treatments are currently available. The agents that have been approved and globally marketed for Huntington disease only help to alleviate the symptoms.
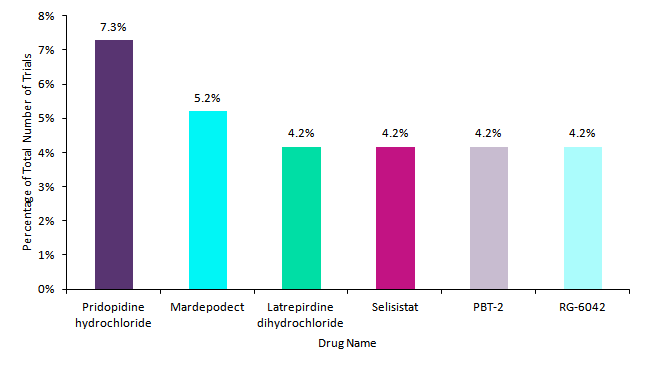
Investigated agents in Huntington disease clinical trials
GlobalData analysed the number of Huntington disease clinical trials with a start date between 6th August 2009 and 6th August 2019.
Within the last 10 years, Teva Pharmaceutica’s pridopidine hydrochloride was the most frequently investigated agent (7.3%) in Huntington disease trials. The drug aims to support the development of neurons through the increased production of brain-derived neurotrophic factor (BDNF). In Huntington disease, BDNF levels are decreased. However, pridopidine hydrochloride failed to meet its primary endpoints in a Phase II trial conducted by Teva, leading to the withdrawal of the planned Phase III trial and discontinuation of the drug candidate.
Pfizer’s mardepodect was the second most investigated agent within this period (5.3%). However, similar to pridopidine hydrochloride, Pfizer decided to discontinue mardepodect due to the lack of efficacy exhibited in a Phase II trial. At this stage, the drug was unable to meets its primary endpoint.
Pfizer’s latrepirdine dihydrochloride, Siena Biotech’s selisistat, Alterity Therapeutics’ PBT-2, and Roche’s RG-6042 finished making up the top six drugs with an equivalent number of clinical trials (4.2%). Latrepirdine dihydrochloride aims to protect brain cells from damage and enhance survival through the stabilisation and improvement of mitochondrial function. However, following the trend of so many Huntington disease prospects, it failed to show adequate efficacy in Phase III trials. As such, its development was discontinued by Pfizer.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataPromising therapies
Siena Biotech’s selisistat displays protective activity against the toxicity induced by huntingtin. In a Phase II study conducted in 2011, selisistat displayed a borderline modulating effect on the concentration of huntingtin. However, clinically relevant changes were not observed in the short study. As selisistat failed to produce clinically relevant effects, the drug’s development remained inactive until 2017, when AOP Orphan Pharmaceuticals AG acquired the rights for selisistat from Siena Biotech with the aim to resume its development.
Alterity Therapeutics’ PBT-2 is a metal-protein-attenuating compound (MPAC) that aims to inhibit the accumulation of metals inside brain cells, which usually accompanies HD. In a 2012 Phase II study, the agent appeared to display some improvements to cognitive function, but the FDA placed the drug on a partial clinical hold based on a non-clinical neurotoxicology findings from a canine study.
Roche’s RG-6042 proves to be the most promising agent within the Huntington disease research landscape. Targeting the toxic huntingtin protein, it blocks its production. A Phase I/II study of RG6042 yielded extremely promising results, with RG-6042 leading to significant decreases in mutant huntingtin (mHTT) of up to 60%.
Furthermore, RG-6042 also displayed an adequate safety profile with no serious adverse events associated with drug. Unlike the other top HD prospects, RG-6042 has two active trials currently in development (Phase II and Phase III). As such, it has the greatest potential to serve as a viable Huntington disease treatment.