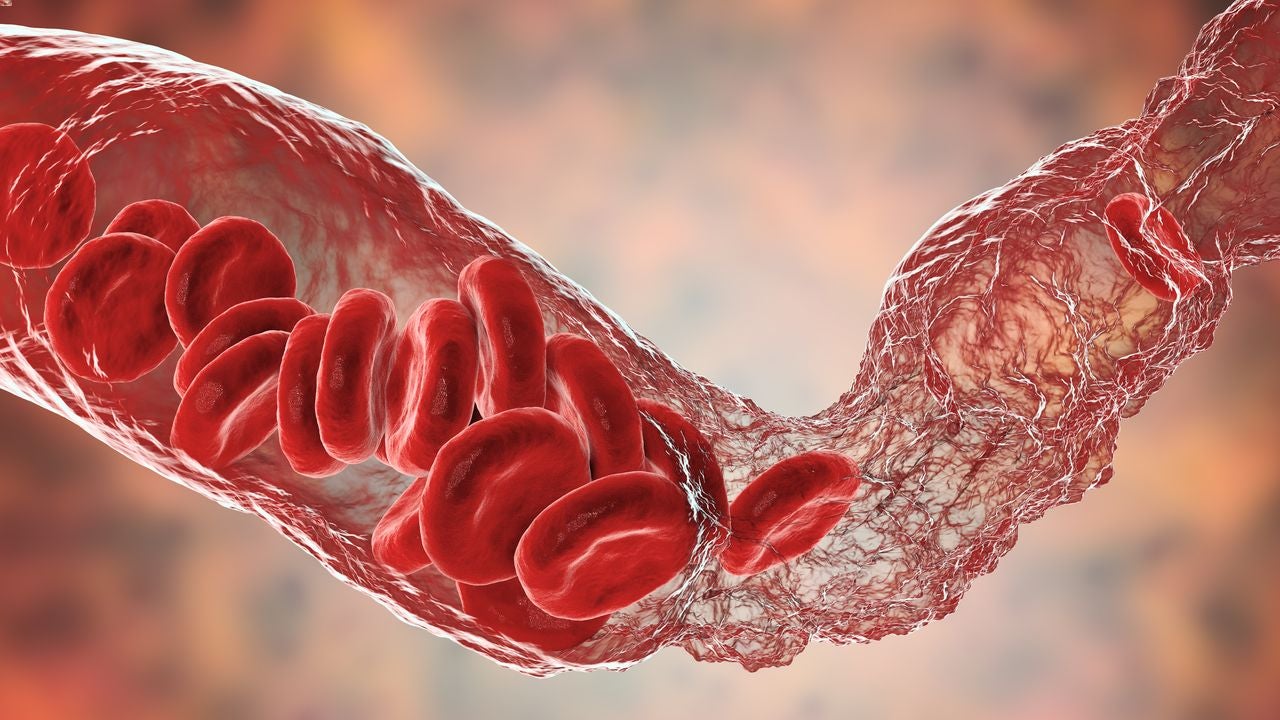
A recent study exploring the risk of venous thromboembolism (VTE) in non-hospitalised Covid-19 patients has found that their absolute risk of VTE is low. Fang and colleagues conducted a cohort study of 398,530 adult outpatients with Covid-19, assessing the rates of VTE in the first 30 days of Covid-19 diagnosis and in follow-up. They found that non-hospitalised Covid-19 patients had a low risk of developing VTE, although this risk was slightly higher in the first 30 days after diagnosis. Additionally, the researchers identified potential factors associated with a higher risk of VTE including being aged 55 years and older, being male, having a history of VTE or thrombophilia, and having a body mass index greater than or equal to 30.0.
VTE is a disease that occurs when a thrombus, or a blood clot, forms in an individual’s deep vein. The VTE may either be provoked, meaning it is caused by specific events such as surgeries or long flights, or unprovoked. VTE can be further segmented into deep vein thrombosis (DVT) and pulmonary embolism (PE). In PE, the thrombus embolises, breaking off and travelling to the lungs. VTE is the third most common cardiovascular condition globally and is associated with long-term mortality and morbidity. The condition can arise in both hospitalised and non-hospitalised individuals and can return, with approximately one-third of patients with a DVT or PE having a recurrence within ten years.
Current treatment strategies primarily consist of administering anticoagulants, namely novel oral anticoagulants (NOACs) such as Bristol Myers Squibb’s Eliquis (apixaban), Janssen Pharmaceuticals’ Xarelto (rivaroxaban), Daiichi Sankyo’s Savaysa (edoxaban), and Boehringer Ingelheim’s Pradaxa (dabigatran). Other anticoagulant options include warfarin, heparin, and fondaparinux. GlobalData identifies one late-stage pipeline therapy that could be promising, Anthos Therapeutics’s abelacimab. This drug is a coagulation Factor XI inhibitor that is administered intravenously or subcutaneously once per month. Abelacimab is said to be promising due to its decreased bleeding risk in comparison to existing treatment options and is also in clinical trial development for atrial fibrillation, ischemic stroke, and inflammation.
Risk factors for developing VTE include age, family history, not moving for long periods of time (such as during a long flight or bedrest after surgery), medical conditions like a clotting disorder, and male sex. Additionally, VTE has been identified as an important complication of Covid-19. Therefore, Fang and colleagues’ findings hold potentially important implications regarding which patient populations should be more intensively monitored for primary prophylaxis.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData