On August 14, TB Alliance became the first nonprofit organisation to receive FDA regulatory approval for its drug, pretomanid, for the treatment of tuberculosis.
This approval followed the successful Nix-TB trial, which took place at three sites in South Africa. With this in mind, a further analysis of the current clinical trial space for tuberculosis can be completed in the Clinical Trials Database of GlobalData’s Pharma Intelligence Center. In this article, a small number of Phase 0, Phase I/II, Phase II/III, and Phase III/IV trials were combined with Phase I, Phase II, Phase III, and Phase IV trials, respectively.
Results of the analysis
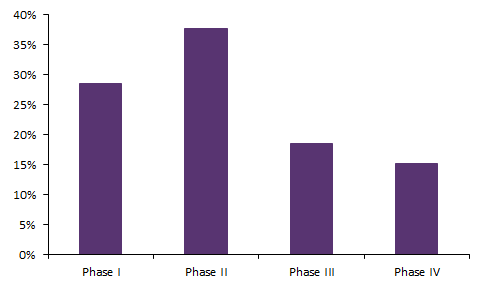
Looking at all of the tuberculosis clinical trials in the database, most trials (37.8%) are in Phase II, as seen in Figure 1. Additionally, 28.5% are in Phase I, 18.6% are in Phase III, and 15.1% in Phase IV. Based on trial status, most trials were completed (66.3%). This was followed by ongoing, recruiting (13.1%); suspended, terminated, and withdrawn (S/T/W) (9.0%); planned (7.9%); ongoing, not recruiting (3.1%); and ongoing, recruiting by invitation (0.5%) (Figure 2).
As stated earlier, the pivotal Nix-TB trial took place in three sites. This is consistent with the majority of the clinical trials in the database. A total of 82.7% of the clinical trials took place at 1–10 sites, 6.3% took place in 11–25 sites, 1.3% took place in 26–50 sites, and only 0.5% took place in more than 50 sites. This can be seen in Figure 3.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData