The Biosecure Act was left out of a key US defence bill that passed in the House last week, in a major win for Chinese CDMOs. Shares in WuXi AppTec (Shanghai, China) opened 10.5% higher on December 9 compared to the previous market close while shares of several Indian CDMOs fell when the news was revealed. If passed, the law would obstruct US companies from outsourcing to certain Chinese biotech providers.
Many pundits had believed that Biosecure would be added to the text of the 2025 National Defense Authorization Act. There is still a slim chance that the Biosecure bill could be added to a Continuing Resolution by 20 December —an emergency bill that extends funding to federal agencies for the first three months of 2025, giving Congress more time to enact a full FY 2025 budget. [However, text of the Continuing Resolution introduced by lawmakers on 17 December omitted any mention of Biosecure, firmly pushing any vote on the legislation to 2025.]
WuXi AppTec and WuXi Biologics (Jiangsu, China) are looking to sell off sites in the US and Europe as the prospect of the Biosecure Act is deterring potential clients, the Financial Times reported on 3 October 3, 2024. This provides opportunities for the Chinese CDMOs’ competitors to both acquire sites and to pick up new business. [WuXi did not respond to our enquiry as to whether it will continue to explore these sales in light of the end-of-year reprieve.]
The Biosecure Act passed in the US House of Representatives in September but has yet to make it through the Senate. If it becomes law, it will prohibit US federal funding for services provided by biotechnology companies of concern such as WuXi AppTec. WuXi AppTec’s Q3 2024 results show very high reliance on the US (64% of revenues). Its Q3 growth declined by 2% year-on-year, driven by a reduction in Covid-19 products.
The news of the reprieve from Biosecure passing this year has reversed prospects for Indian CDMOs, who until early December had been reporting an increase in inquiries as pharma clients become deterred from using Chinese services. Hikal Ltd’s (Maharashtra, India) Manoj Mehrotra, president of the pharmaceutical business, stated in an earnings call on 12 November 2024: “We are getting more inquiries and especially from the US customers because of this Biosecure Act […]. They are all innovative customers [and] are looking more towards India in the last year.” Stock prices of Divi’s Laboratories (Telangana, India), Neuland Laboratories (Telangana, India), Piramal Pharma (Maharashtra, India), and Syngene International (Karnataka, India) dropped in response to the Biosecure Act being left out of the key defense bill this month.
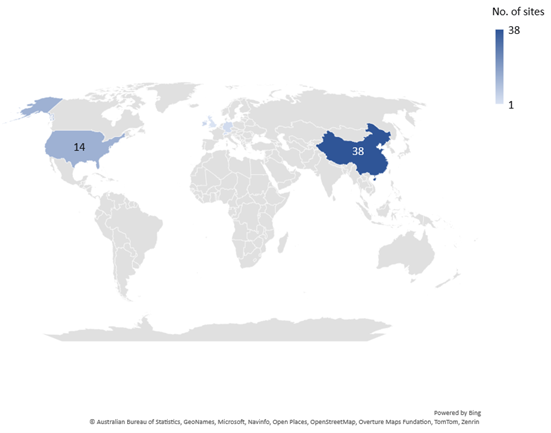
Potential WuXi manufacturing site sales
In a Q3 2024 earnings call on October 29, 2024, WuXi AppTec stated that its ATU cell and gene subsidiary has already been impacted as there were “insufficient new business wins due to the proposed US legislation.” In a separate press release on 8 October 2024, WuXi AppTec stated: “There is uncertainty as to whether the Company will eventually decide to dispose [of] WuXi ATU (Jiangsu, China), or take other options to retain WuXi ATU and execute other options so that such business can continue to operate.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe Financial Times reported that affiliated company WuXi Biologics is in talks to sell some of its European production facilities, as demand has dried up. This reportedly includes two German manufacturing sites WuXi Biologics previously acquired from Bayer (North Rhine-Westphalia, Germany).
While the majority of various WuXi subsidiaries’ combined pharma manufacturing sites are in China, they own 14 in the US, four in Ireland, three in Germany, two in Switzerland, and one in the UK.
WuXi AppTec stated that it “strongly disagrees with any preemptive and unfair designation of us as a named “biotechnology company of concern” without due process. The company fully complies with the laws and regulations in the countries and regions in which we operate”.
Update: The third and fourth paragraph were updated with information on the latest status of the Continuing Resolution as of 17 December.