Global drug supply chains could be further disrupted as foreign pharma inspectors shared that they fear arrest after China tightened its anti-espionage laws, and are receiving refusals to share documents.
The German Medicines Manufacturers’ Association (BAH) claims that many German inspectors are refusing to visit China after the government increased its powers to fight espionage in April 2024. This law is vaguely worded, making it possible that any information gathering could be criminalised, including activities carried out as part of an audit or inspection. Espionage suspects can be prevented from leaving China and that also applies to foreign nationals.
Although no Western pharmaceutical inspectors have been arrested for spying to date, a Japanese executive from Astellas Pharma (Tokyo, Japan) was arrested last year in China on accusations of espionage. The arrest aroused fear among foreign companies and individuals who are now more cautious about their Chinese operations.
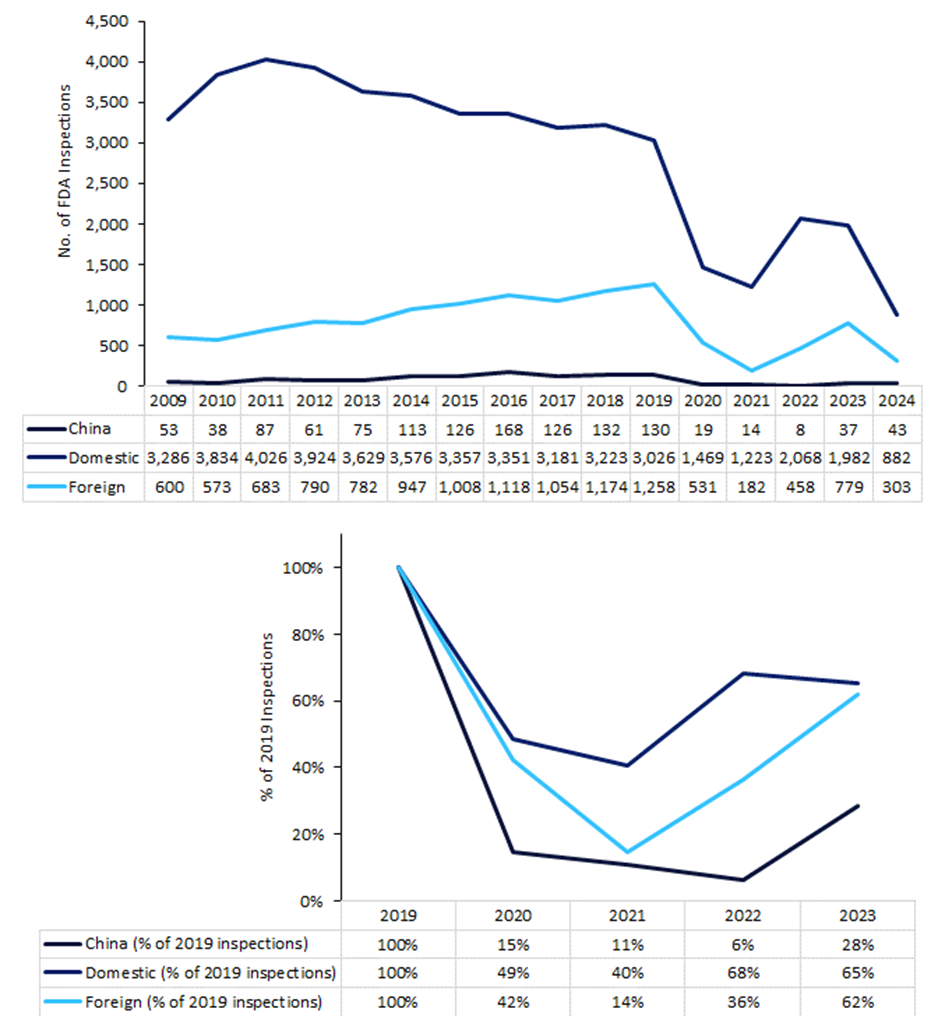
At other times, it is the manufacturers themselves that are preventing inspections. Inspectors from the US Food and Drug Administration (FDA) have reported an increase in refused entry to Chinese production sites since the Covid-19 pandemic. Data from the FDA shows that the agency’s inspectors began to be turned away from Chinese factories in 2021, the German newspaper Handelsblatt reports. In total, there have been 150 instances of refused FDA inspections since then. There were 26 inspection refusals last year, down from a peak of 62 in 2022. Under Section 704 of the FD&C Act, if a site delays, denies, limits, or refuses an inspection, the drugs may be deemed adulterated. A reduction in inspections and audits will further worsen supply chain issues and shortages of medicines globally, as EU and US regulators require imported drugs to have GMP certification for their production facilities.
Chinese site inspections plummet since pandemic
At the beginning of the Covid-19 pandemic, the FDA halted inspections of most overseas and domestic drug manufacturing establishments. Figure 1 (below) shows that even now, the volume of FDA inspections worldwide has not returned to pre-pandemic levels, but in China resumption lags far behind other regions. In attempting to inspect foreign sites, the FDA has been troubled by jurisdictional issues, language barriers, and travel clearance requirements.
Inspections of Chinese facilities in 2020 dropped to 15% of 2019 levels and rose to only 29% by 2023. Recovery in other regions was swifter, reaching 42% of 2019 levels (all foreign countries), 49% (in the US) by 2020, and 62% (foreign) and 66% (in the US) by 2023.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe top citations (2009–2024) for Chinese facilities were for problems related to laboratory controls and failure to follow or absence of written procedures.
Figure 1: FDA inspections of drug and biologic facilities, 2009–2024