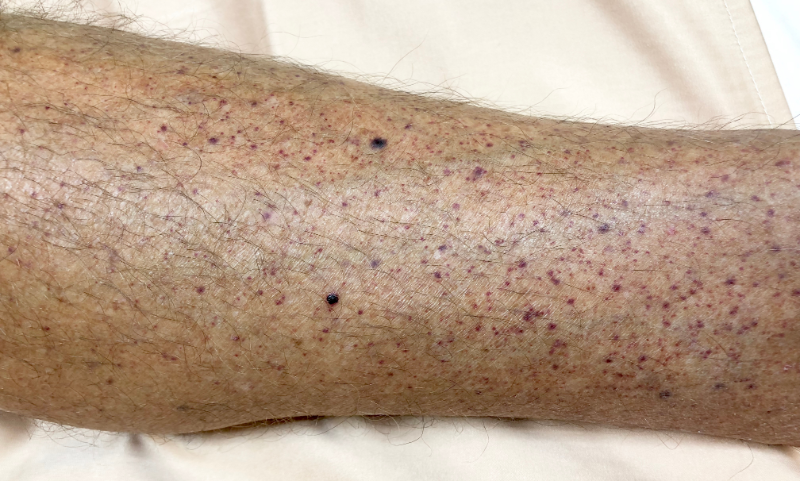
Idiopathic thrombocytopenic purpura is a haematological disorder that is characterised by the production of antibodies that bind to platelets, causing symptoms such as epistaxis, menorrhagia in women, and the formation of petechiae. Severe complications can include intraperitoneal or intracerebral haemorrhage, which most frequently occurs when the platelet count is less than 5,000 per μl. The condition is normally managed with corticosteroids such as dexamethasone and prednisone, intravenous immunoglobulins, or thrombopoietin receptor agonists such as Novartis’ Promacta (eltrombopag), and Dova Pharmaceuticals’ Doptelet (avatrombopag), which are associated with side effects such as myalgia, oedema, pruritus, and insomnia.
There are five first-in-class products in clinical development for the treatment of idiopathic thrombocytopenic purpura. These drug candidates target proteins that are not acted upon by any commercially available marketed drugs. Most (60%) of the first-in-class products are being manufactured by companies in the Asia-Pacific region. These include a Japanese biopharma company, KM Biologics, which is manufacturing apadamtase alfa; an Australian biotech company, CSL, which is manufacturing CSL-730; and batoclimab, a monoclonal antibody that is being developed by the Chinese company Harbour BioMed and the South Korean company HanAll Biopharma. The most promising of these drug candidates is batoclimab, which has met efficacy, safety, and pharmacokinetic endpoints in multinational clinical trials. The presence of first-in-class products in the pipeline indicates a deeper understanding of the pathophysiological mechanisms that drive disease progression. Furthermore, increased R&D that focuses on rare diseases such as idiopathic thrombocytopenic purpura is expected to yield positive financial results, as the market is expected to be worth more than $2.4B by 2026. Drug candidates for this indication also benefit from being awarded orphan drug designation by the FDA for their potential to better serve the needs of refractory patients.
Research has shown that patients from countries in the Asia-Pacific region differ in disease presentation and response to therapy compared to their European and North American counterparts. For example, there is a greater need for steroid-sparing treatment regimens in the Asia-Pacific region because of the link between corticosteroids and gastrointestinal side effects such as gastritis, abdominal distention, dyspepsia, and constipation. This is significant for idiopathic thrombocytopenic purpura patients in the Asia-Pacific region because of the increased prevalence of Helicobacter pylori (H pylori) infections in the gastrointestinal tract. Infection with H. pylori is associated with the development of peptic ulcers, which can bleed and cause further complications. Therefore, it is understandable that companies in the Asia-Pacific region are spearheading innovation in the pipeline for idiopathic thrombocytopenic purpura, as first-line, second-line, and third-line therapies have all been linked to negative gastrointestinal outcomes.
While the late-stage pipeline does include first-in-class products, idiopathic thrombocytopenic purpura remains a life-long condition that affects patients in a relapsing-remitting pattern that tangibly contributes to a decreased quality of life. For now, patients will need to continue to take extreme precautions to reduce the risk of significant blood loss from minor incidents. However, these measures may be a temporary solution if pipeline therapies show superior efficacy and safety profiles compared to commercially available marketed drugs.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData