Regulatory authorities play a pivotal role in accelerating, encouraging and incentivising the development and approval of novel treatments via the awarding of review designations. Since the first review designation was awarded in 1984, the US Food and Drug Administration (FDA) has overseen a significant rise in the annual number of designations granted, culminating in 694 designations in 2020. Key pharmaceutical events, including the boom of the biotech industry and the advent of groundbreaking scientific technologies, have caused this trend in review designation awarding over the last 40 years.
Regulatory authorities play their part in expediting drug development and approval through the awarding of review designations. To date, the FDA has implemented seven different types of review designations, which accelerate and incentivise drug development. Each designation offers specific benefits to the drugmakers. For example, orphan drug designation (ODD) is granted to drugs intended to treat a rare condition providing tax credits for qualified clinical trials, exemptions from user fees and a potential of seven years market exclusivity following approval.
Trends in FDA review designation awarding can be seen in Figure 1. The FDA awarded its first 22 designations, consisting solely of ODD, in 1984. From 1984 to 1997, it awarded an average of only 23 designations per year, indicating limited adoption of review designations.
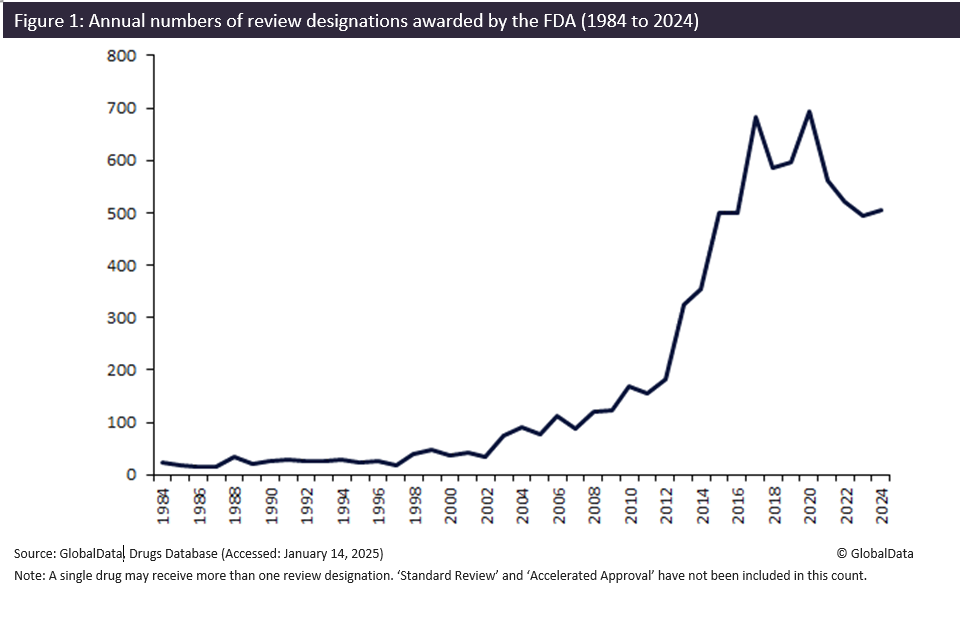
From 1998 to 2012, the FDA experienced an increase in awarding. During this period, an average of 93 designations were awarded yearly, and in 2012, 182 review designations were awarded – more than 10 times the number awarded in 1997. This finding is aligned with the Modernization Act of 1997, in which the FDA sought to speed up the approval of new drugs which saw the introduction of the fast-track and priority review designation types in 1998 and 1999, respectively. This period also coincided with the advent of the biotech industry, as the 2003 completion of the human genome project catalysed gene therapy developments.
2012 to 2020 saw another rapid increase in the annual number of review designations awarded. Over this period, annual designations awarded by the FDA increased almost four-fold. The period included the implementation of novel designation types: qualified infectious disease product designation (in 2012), breakthrough therapy (2013), rare paediatric drug designation (2014) and regenerative medicine advanced therapy designation (2017). The introduction of these specific designation types reflects the booming biotech industry, and the embrace of novel technologies to develop advanced biologics, which began entering clinical development.
In 2020, a peak of 694 designations was awarded. This peak reflected heightened pharmaceutical activity and investment during the Covid-19 pandemic, which accelerated the development of critical therapies and vaccines. Since 2020, the number of review designations has declined, with only 506 awarded in 2024. This decline is attributed to a return to post-pandemic normality, whilst still maintaining historically high levels of activity compared to earlier decades.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataSince its introduction in 1984, the FDA has overseen increases in the annual awarding of review designations. This trend mirrors the evolution of the pharmaceutical industry, in terms of investment and utilisation of novel technologies. Today, seven review designations remain an integral part of the FDA’s toolkit, expediting the approval of critical therapies and ensuring that life-saving drugs reach patients as quickly as possible. For further insight into the trends of pharmaceutical review designations, please see the recently published report: Pharmaceutical Review Designations: Trends and Industry Insights.





