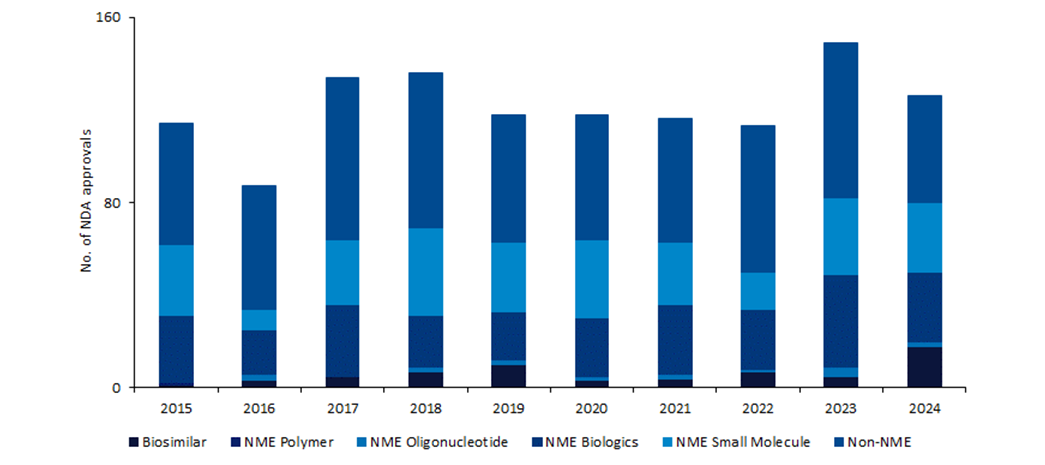
The US Food and Drug Administration (FDA) approved 126 innovator and biosimilar drugs in 2024, significantly lower than the 149 approvals in 2023. However, there was an abundance of innovation and firsts among 2024’s approvals.
Numerous small pharma companies secured their first market approval in 2024 such as Madrigal Pharmaceuticals’ (Conshohocken, US) Rezdiffra, the first treatment for patients with liver scarring due to fatty liver disease, and Verona Pharma’s (London, UK) Ohtuvayre, for chronic obstructive pulmonary disease in adult patients. Smaller pharma companies such as these tend to be more reliant on contract manufacturing organisations for the manufacture of their product.
Approvals last year included rare disease treatments, new oncology drugs, Eli Lilly’s (Indianapolis, US) Kisunla, an amyloid-targeted Alzheimer’s drug, and the first mesenchymal stromal cell therapy approval, Mesoblast’s (New York City, US) Ryoncil.
The Center for Drug Evaluation and Research identified 24 (48%) of the 50 novel drugs (new molecular entities or new biologics) approved in 2024 as first-in-class, a higher percentage of first-in-class approvals compared to 2023, which accounted for 36% of approvals. These drugs have mechanisms of action different from those of existing therapies. New and innovative products tend to require a high level of expertise and sophisticated manufacturing equipment, which increases the likelihood they will be outsourced. Notable first-in-class drugs included:
- Altor Bioscience’s (Miramar, US) Anktiva intravesical solution (medications placed directly in the bladder through a catheter) to treat non-muscle-invasive bladder cancer with carcinoma in situ with or without papillary tumours that are unresponsive to prior therapy with Bacillus Calmette-Guérin.
- Bristol Myers Squibb’s (Princeton, US) Cobenfy capsules, the first muscarinic-acting drug to treat schizophrenia in adults.
- Syndax Pharmaceuticals’ (Waltham, US) Revuforj tablets and oral solution to treat relapsed or refractory acute leukaemia with a lysine methyltransferase 2A gene translocation in patients aged one and older.
- Idorsia Pharmaceuticals US’ (Wilmington) Tryvio tablets to treat hypertension (high blood pressure) in combination with other antihypertensive medications, to lower blood pressure in adults who are not adequately controlled on other medications.
For a greater level of analysis related to drugs that were marketed in 2024 and their manufacturing arrangements, please refer to the upcoming PharmSource report New Drug Approvals and Their Contract Manufacture – 2025 Edition.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData