The complement cascade has been implicated in the pathology of a number of different neurological diseases, from autoimmune diseases like myasthaenia gravis (MG) to neurodegenerative diseases like amyotrophic lateral sclerosis (ALS). At the American Academy of Neurology (AAN) 2022 Annual Meeting, new data were presented for several complement-targeting therapeutics, which highlight the potent efficacy of this approach both in controlled trials and in the real world. The agents featured at AAN 2022 are promising and represent only a fraction of the growing list of complement-targeting agents in development for neurological indications.
The first complement-targeting therapy approved for the treatment of neurological diseases was AstraZeneca/Alexion’s Soliris (eculizumab), approved in 2017 in the US for anti-acetylcholine receptor antibody positive (AchR+) generalized MG and in 2019 for aquaporin-4 antibody positive (AQP4+) neuromyelitis optica spectrum disorder (NMOSD). Soliris is an intravenously (IV) delivered monoclonal antibody (mAb) that binds complement component 5 (C5), preventing its cleavage into C5a and an C5b; C5a acts as a chemotactic agent and anaphylatoxin while C5b serves as part of the membrane attack complex, whereby cytotoxic pores are formed across cell membranes. While this innate immune pathway is useful in killing bacterial cells, when directed toward self, it can cause cytotoxic damage and associated pathology.
New data presented on Soliris at AAN 2022 included three presentations on real-world experiences in patients with MG and two studies focusing on NMOSD. For MG, data was presented from an MG registry in the US, a retrospective chart review study in the US (Project ELEVATE), and a post-surveillance study in Japan, all of which were sponsored by AstraZeneca. All studies demonstrated that patients initiating Soliris in a real-world setting had demonstrated strong improvement over time. For example, in the presentation of data from Project ELEVATE, the mean MG-activities of daily living (MG-ADL) score dropped significantly from 8.0 prior to Soliris initiation to 3.7 after only six months and was sustained through 24 months. Additionally, among patients receiving prednisone at Soliris initiation, 77% were able to either discontinue or reduce their dosage.
For NMOSD, interim data was presented from a Japanese post-marketing surveillance study that showed a marked decline in the incidence of relapses among patients with NMOSD after initiating Soliris: in the two years prior to initiating they therapy, 36 patients experienced 76 relapses versus zero relapses over 36 weeks of treatment on average. This finding on relapse prevention was supported by another presentation comprising an indirect analysis of published data from randomized controlled studies of FDA-approved treatments for AQP4+ NMOSD. Specifically, Soliris was found to be more effective in preventing relapses compared to Roche’s Enspryng (satralizumab) or Horizon Therapeutics’ Uplinza (inebilizumab).
In 2021, AstraZeneca filed for FDA approval of a second-generation C5-targeting mAb, Ultomiris (ravulizumab) in AchR+ generalized MG. On April 28, 2022, the drug was granted approval. Although it utilizes the same MOA as Soliris, Ultomiris boasts less frequent administration (once every eight weeks rather than every two weeks) due to slower clearance from the body. Pharmacokinetic and pharmacodynamic data presented at AAN 2022 support the long-lasting potency of Ultomiris, hopefully meaning the drug will be able to address an important unmet need in MG—treatment burden and frequency. Ultomiris in an IV formulation was recently approved, but earlier-stage studies for a subcutaneous formulation are ongoing, which could offer patients an additional treatment option in the future.
Topline results from the Phase III study of Ultomiris in MG, CHAMPION MG (NCT03920293), were announced in July 2021, suggesting the drug met key primary and secondary endpoints. Results presented at AAN 2022 bolstered these claims by providing more granular data on efficacy and safety during the placebo-controlled portion of the trial as well as data from the associated open-label extension (OLE) study. During the placebo-controlled portion of the trial, significant improvements in MG-ADL scores were observed within one week and were sustained through week 26; data from the OLE study showed that these improvements persisted to the end of the 60-week study period. Similar patterns of efficacy were seen for several secondary endpoints, including the Quantitative Myasthenia Gravis total score and two quality of life measures, over the course of the placebo-controlled and OLE portions of the study. Across both portions of the CHAMPION study, no new safety concerns were reported, with only five patients (3.0%) experiencing a serious adverse event deemed related to Ultomiris.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn addition to the two approved complement-targeting agents described above, GlobalData has identified 28 additional agents in clinical or preclinical phases of development for neurological conditions. These include three products in Phase III, four in Phase II, five in Phase I, and 16 in preclinical or discovery phases. Figure 1 captures the complement-targeting pipeline products in development across neurological indications.
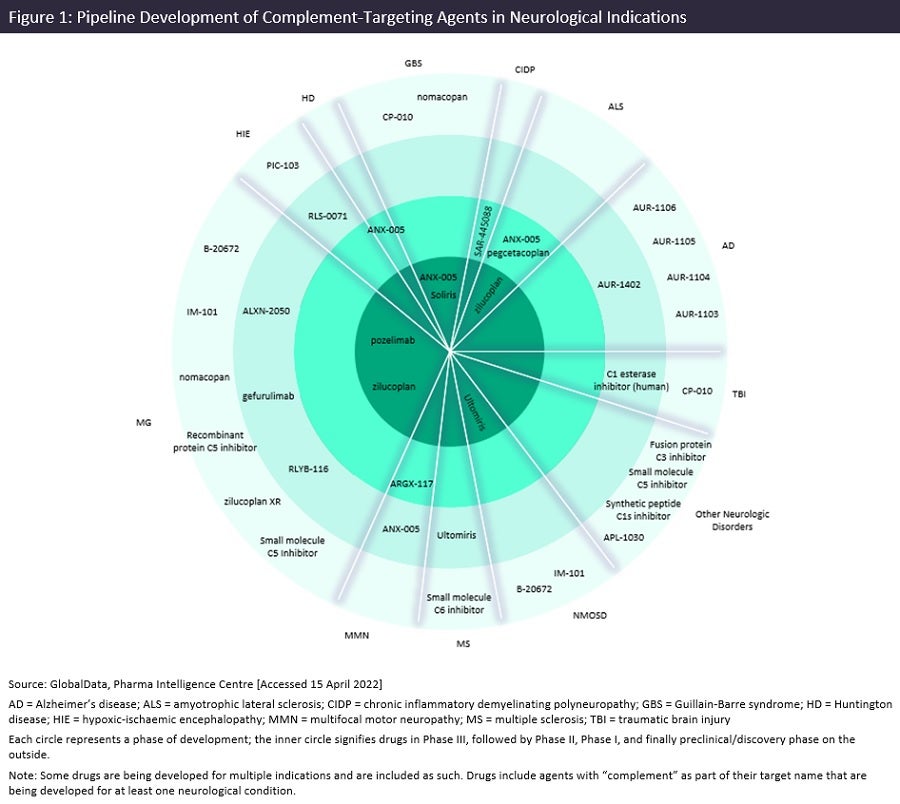
MG is the neurological indication with the greatest number of complement-targeting agents in development with eleven agents, followed by AD with five in development, albeit mostly in preclinical/discovery stages. In regards to the specific complement pathways targeted, 23.3% of the pipeline products in development utilize the classical complement pathway (C1s/C1q and C2), 66.7% utilize the alternative pathway (C5, C6, C9, and Factor D), and 10.0% target C3, which plays roles in both the classical and alternative complement cascade. Phase III products currently in development include UCB’s zilucoplan for ALS, Regeneron Pharmaceutical’s pozelimab for MG—both C5 targeting agents—as well as Annexon Inc’s C1q inhibitor, ANX-005, for GBS. ANX-005 was the only of these three that had data presented at AAN 2022; presentations included data on a small trial exploring ANX-005 in combination with IVIG (NCT04035135) while another explored the impact of ANX-005 on classical complement activation in CSF fluid collected from patients participating in a placebo-controlled Phase Ib study.
With promising data presented at AAN 2022 and a consistently broadening pipeline, targeting the complement cascade is a neuroimmunological strategy of growing importance across a wide range of neurological conditions. GlobalData expects these agents to have a significant impact on the market going forward.




