Astellas Pharma unveiled its plans to construct an active pharmaceutical ingredient (API) manufacturing facility in Toyama, Japan, in January 2020.
Provisionally the third fermentation building, the facility will be used to manufacture API of Astellas’ Prograf® (tacrolimus hydrate), an anti-rejection medicine for liver, kidney and heart transplant patients.
Construction of the API manufacturing facility will begin in April 2020 and completion is expected in August 2021.
Third fermentation building is anticipated to cost approximately JP¥10bn ($90.8m).
Astellas new manufacturing facility location
Astellas’ Toyama Technology Centre in Toyama, the capital city of Toyama prefecture in Japan, will be the location of the proposed manufacturing facility.
The site extends across an area of 191,000m² and includes a built-up area of 23,986m².
Details of Astellas new manufacturing facility
The API manufacturing facility will offer a total floor space of 7,220m² and include three floors above the ground level, equipped with advanced production facilities.
Third fermentation building will help increase the company’s production capacity to meet the projected demand for Prograf and ensure the steady supply of products.
Toyama Technology Centre details
Toyama Technology Centre commenced operations in 1992 and is involved in the production and packaging of Prograf capsules and granules and Protopic ointments. It manufactures Prograf APIs for Japan and overseas markets.
Prograf and Protopic products are used as medications for immunological diseases. The centre also houses research facilities, and the company has plans to add facilities in future to manufacture bio-investigational drugs and commercial products.
Details of Prograf
Prograf is a calcineurin-inhibitor immunosuppressant prescribed for use with separate medicines to prevent organ rejection in patients receiving kidney, liver or heart transplants. It gained initial approval from the US FDA in 1994.
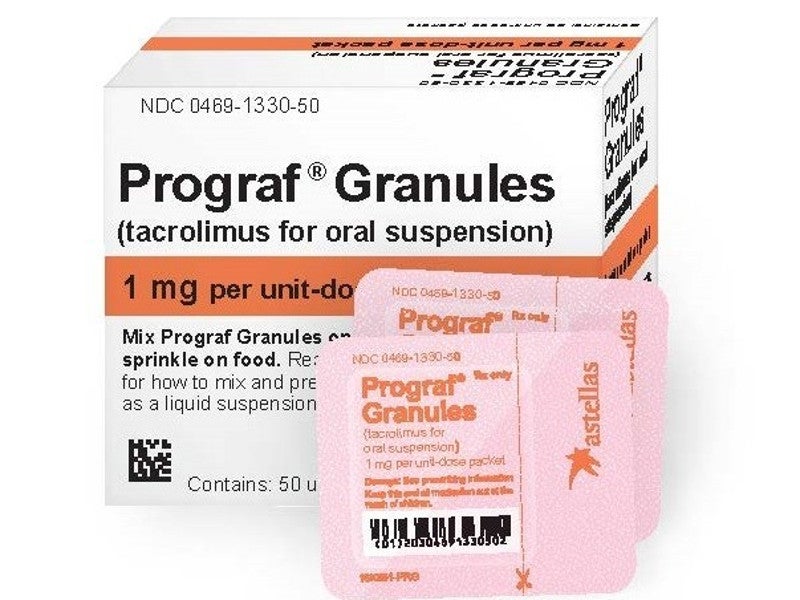
Developed by Astellas Pharma, Prograf capsules and granules are tacrolimus immediate-release drugs used for oral administration. In March 2019, the company launched Prograf Granules in the US as a therapeutic alternative for paediatric patients receiving organ transplantation. The firm supplies the Prograf immunosuppressant to approximately 100 countries.
Other brand names of tacrolimus hydrate offered by the company are Advagraf® Graceptor® and Astagraf XL®.
Prograf granules are available in standardised packets of 0.2mg or 1mg containing 50 units for facilitating precise dosing in paediatric patients. The product is also accessible as 5mg / ml injections for intravenous use.
Marketing commentary on Astellas Pharma
Astellas Pharma is an international pharmaceutical company engaged in the manufacturing and marketing of pharmaceuticals, focused on providing innovative and reliable pharmaceutical products to improve the health of people.
Established in April 2005, the company is headquartered in Tokyo and employs 16,243 people as of March 2019.
Astellas Pharma follows a focus area approach to develop innovative medicines for diseases with unmet medical needs. It offers numerous products for the treatment of diseases, such as overactive bladder (OAB), acute myeloid leukaemia (AML), prostate cancer and benign prostatic hyperplasia.
Astellas invests in research and development activities to bring breakthrough discoveries to patients. In addition to its R&D efforts, the company collaborates with biotechnology companies and academic research institutes.
Key therapeutic areas of interest include oncology, nephrology, neuroscience, immunology, urology, ophthalmology, muscle diseases, regenerative medicines, vaccines and gene therapy.
Immuno-oncology, mitochondrial function, antigen-specific immunomodulation and regenerative medicine are the four areas of primary focus at the company’s innovation sites in Cambridge and Marlborough, US.
The company operates through locations in North America, South America, Europe, Middle East and Africa (EMEA) and Asia-Pacific regions. It closed the acquisition of Adeno-associated viral vector (AAV)-based genetic medicines firm Audentes Therapeutics, in January 2020.