In April 2001 Eli Lilly announced it was constructing a new biotech bulk manufacturing facility in Carolina, Puerto Rico. Construction started in July 2002 and, following validation and commissioning, the plant was in production by mid-2006. This project was part of an overall $1bn investment into the company’s facilities in Puerto Rico, which was completed in August 2006.
The 300,000ft² facility produces the rapid-acting insulin product Humalog, dispensed from the KwikPen (MirioPen), which is manufactured in Indianapolis.
The production of this requires the use of recombinant DNA technology via insulin lispro [rDNA origin] injection. KwikPens became available for Humalog, Humalog Mix75/25 and Humalog Mix50/50 in February 2008.
KwikPen is the third new insulin pen launched since 2007 to improve the daily management of diabetes. Previous models include the HumaPen MEMOIR, the world’s first digital insulin pen with memory; and HumaPen LUXURA HD, a reusable pen for patients who need insulin dosing in smaller increments.
In February 2010, the plant was issued a warning letter by the US Food and Drug Administration (FDA) for deviations in cGMP API production. The FDA cited deviations during an inspection conducted in 2009. Deviations were found in 24 batches of Lyspro Insulin Zinc Crystals, the API in Humalog, released between December 2007 and March 2008.
Production of the original Humalog pre-filled pens including the Humalog Mix75/25 and the Humalog Mix50/50 was discontinued from 2011. These pens have been replaced by the KwikPen device.
Facility and production
The facility was initially planned to cost $250m. However, the construction design was expanded to include a dedicated warehouse facility, materials storage, a utility building, an administration building, dispensing centre, a science and technology laboratory, training facilities and an independent laboratory facility. These are contained in a four-storey, 50,000ft² building for the site, increasing the total investment to $450m and raising the number of new employees from 300 to 450. These facilities were ready in the first quarter of 2007.
It was important for Eli Lilly to get the plant into production as quickly as possible as the world insulin market is growing daily with the number of diabetics increasing, and new long-acting insulin products such as Humalog are in demand. The World Health Organization (WHO) has predicted that the number of people with diabetes will increase from 135 million to 300 million over the next 25 years.
Insulin / Humalog production
The plant consists of five operational suites. The first is the media preparation area where the bulk media is formulated before inoculation and up-filling to the pre-fermentation vessels to begin the fermentation.
The second is the fermentation suite (consisting of three 15,000L bioreactors) and associated storage tank farm.
The third is the pre-purification area where the Escherichia coli are killed and broken down (lysed) to harvest the pre-proinsulin. The pre-proinsulin is separated out from the cell debris by centrifugation and filtration.
The fourth area is the refolding suite where the pre-insulin is treated with buffers to assist it in attaining its tertiary structure. The pre-proinsulin may still be attached to other peptides added to it by the E. coli so further purification is required.
The final area is the downstream purification suite where the pre-proinsulin is cleaved using trypsin to modify its primary structure and then separated by chromatography and finally crystallised.
The chromatographic process is monitored by protein-specific analysis using enzyme-immunological methods that make it possible to detect even the smallest possible by-products. The purity of the insulin is measured at every intermediate stage of production by the In Process Control (IPC) laboratory.
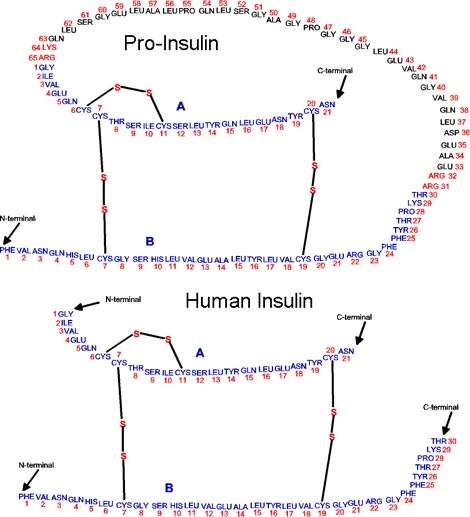
The product insulin (lispro) can then be used to formulate Humalog, which is a mixture of insulin lispro and insulin lispro protamine.
Humalog is a fast-acting insulin which can be administered in a variety of forms – via injection and from an insulin pen injector. It may soon be available to inhale.
Humalog was developed when it was discovered that the time action of insulin could be accelerated by changing the order of two amino acids in the human insulin molecule. It was approved in the US in 1996. Humalog is designed to mimic the body’s natural rapid insulin output in response to eating a meal and acts 15 minutes after injection rather than 45–60 minutes with other insulin injections.
Contractors for the Humalog plant
The architects for the plant were Caribbean Architects and Engineering. The lead contractors responsible for overall project management and civil engineering were Fluor Daniel. The contractor responsible for the fabrication and erection of the structural steel in the new buildings of the facility was Cives Steel Company of the US.
PACIV were responsible for the automation of the plant and the control systems along with instrument validation. CPI Engineers were the consultants for the process engineering in the new plant.
The process piping and the installation of the plant equipment was undertaken by Kinetic Systems Caribe of Vega Alta, Puerto Rico. The cleanrooms in the facility were provided and installed by the McIlvaine Company of Illinois, US. CREW provided technical services and on-site logistics capability.
Recombinant DNA technology
The new plant uses recombinant DNA (rDNA) technology to produce insulin by a cell-based fermentation method. Human recombinant insulin is produced by inserting the insulin gene into a suitable vector. The most readily amenable is a non-pathogenic weakened strain of the common bacterium E. coli. The bacteria produce insulin that is chemically identical to its naturally produced counterpart.
Human insulin is the only animal protein to be made in bacteria in such a way that its structure is absolutely identical to that of the natural molecule. This reduces the possibility of complications resulting from antibody production.
In chemical and pharmacological studies, commercially available rDNA human insulin has proven indistinguishable from pancreatic human insulin. Initially the major difficulty encountered was the contamination of the final product by the host cells, increasing the risk of contamination in the fermentation broth. This danger was eradicated by the introduction of purification processes.
The entire procedure can now be performed using yeast cells as an alternative growth medium, as they secrete an almost complete human insulin molecule with perfect three-dimensional structure. This minimises complex and costly downstream purification procedures.
Eli Lilly in Puerto Rico
Eli Lilly already had a large presence in Puerto Rico with three other plants; two in Carolina manufacturing APIs for Prozac, Darvocet and Axid with a form-fill-seal facility, and one fermentation plant in Mayaguez that produced bulk antibiotics.
Eli Lilly, operating as its Caribbean subsidiary Lilly del Caribe, therefore was able to draw on an already established base of skilled employees to plan the construction of a major new biotech manufacturing facility.
In addition, the company received government incentives provided by Puerto Rico’s 1998 Tax Incentive Act and US government incentives via Section 936 of the IRS code, which phased out from 1996 to 2006. This created tax breaks for a wide range of economic sectors (including a special R&D tax deduction) as well as initiating a flat corporate tax rate, ranging between 2% and 7%. The Puerto Rican Industrial Development Co (PRIDCO) was also involved in negotiating the deal.
Despite global restructuring by Eli Lilly, Humalog continues to be manufactured in Puerto Rico in bulk and then transported to the US operation for filling into dispensing pens ready for sale.