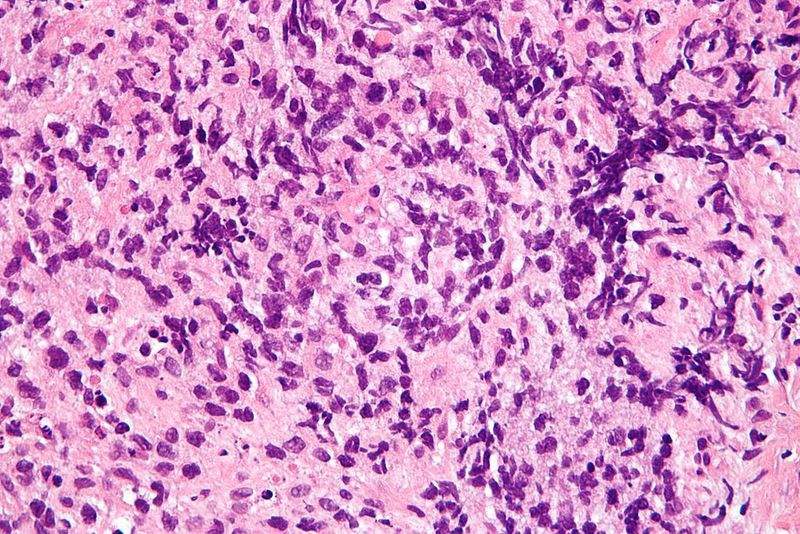
Daiichi Sankyo has secured orphan drug designation from the Japan Ministry of Health, Labour and Welfare (MHLW) for its axicabtagene ciloleucel therapy to treat select B-cell lymphoma types.
The indication allows the use of the drug for diffuse large B-cell lymphoma (DLBCL), primary mediastinal (thymus) large B-cell lymphoma (PMBCL), high-grade B-cell lymphoma (HGBL) and transformed follicular lymphoma (TFL).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Axicabtagene ciloleucel is a chimeric antigen receptor T cell (CAR T) therapy designed to target CD19 and leverage patient’s own immune system against the cancer.
Daiichi Sankyo obtained exclusive rights to develop, manufacture and commercialise the B-cell lymphoma therapy in Japan from US-based Kite Pharma in January last year.
Daiichi Sankyo R&D division Oncology function head Kouichi Akahane said: “Receiving orphan drug designation is an important step in expediting the development of axicabtagene ciloleucel in Japan and underscores the unmet needs of patients with these aggressive forms of relapsed or refractory B-cell lymphomas.
“We look forward to working closely with the Japan Health Authority to bring this important cell therapy to patients in Japan as soon as possible.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAxicabtagene ciloleucel already received approval in the US and Europe based on the data obtained during the Phase I/II ZUMA-1 clinical trial.
According to long-term follow-up ZUMA-1 results reported in December last year, 42% of participants continued to experience a positive response, including 40% of those with a complete remission, at a median of 15.4 months after treatment with axicabtagene ciloleucel.
Daiichi Sankyo is planning to evaluate the B-cell lymphoma therapy in a similar Phase II trial in Japan.




