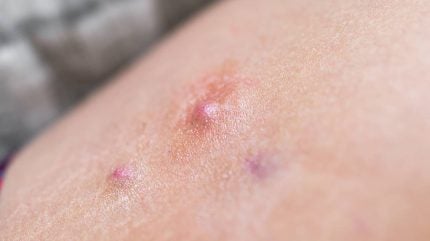
Incyte’s stock price has taken a hit of more than 11%, despite two Phase III trials investigating its oral small-molecule JAK1 inhibitor in hidradenitis suppurativa (HS) hitting their primary endpoints.
The US-based company’s Stop-HS1 (NCT05620823) and Stop-HS2 (NCT05620836) trials hit their primary endpoints with both doses of povorcitinib, 45mg and 75mg, with a higher proportion of patients treated with the therapy. This achieved a Hidradenitis Suppurativa Clinical Response (HiSCR), which is defined as more than 50% from baseline in the total abscess and inflammatory nodule count.
After 12 weeks of treatment, in the Stop-HS1 trial, 40.2% of patients receiving 45mg povorcitinib saw HiSCR versus 29.7% in the placebo group while patients in the 75mg cohort saw similar results with 40.6% achieving HiSCR. Results in the Stop-HS2 trial saw similar rates with patients in the 45mg group seeing 42.3% HiSCR rates versus the same percentile in the 75mg group.
At the same time, the trials met key secondary endpoints, including the proportion of patients achieving a 75% reduction in inflammatory nodule count.
Both studies included a 12-week double-blind, placebo-controlled treatment period, followed by a 42-week extension period and a 30-day safety follow-up period.
Despite this, the company’s stock price has taken a hit of more than 11%, dropping from $67.72 before the announcement, down to $60.72 per share at the time of publication (11am EST, 17 March).
The studies each enrolled approximately 600 children across more than 100 international sites diagnosed with moderate to severe HS for at least three months before a screening visit, who presented with more than five lesions in at least two different anatomical areas.
Incyte’s chief medical officer Steven Stein said: “HS is a challenging and debilitating condition without a cure. Given the limitations of current HS treatments and their impact on patients’ daily lives, there is a critical need for new, well-tolerated and effective therapies that provide a rapid reduction in the signs and symptoms of HS, in particular, pain.
“The positive Phase III data highlights the potential of povorcitinib as an effective oral treatment option for people living with HS.”
Assuming povorcitinib makes it to market, research by GlobalData’s Pharmaceutical Intelligence Center estimates that it is likely to bring in $34m by the end of 2026, with that figure estimated to rise to $625m by the end of 2030.
GlobalData is the parent company of Clinical Trials Arena.
The company is now planning to use these results to advance regulatory submissions worldwide. Away from HS, the company is examining povorcitinib in a Phase III trial in vitiligo, as well as an ongoing Phase II trial in asthma.
Elsewhere in the field of skin conditions, Eli Lilly’s Ebglyss (lebrikizumab-lbkz) has achieved complete skin clearance after three years in 50% of patients with moderate-to-severe atopic dermatitis (AD). Meanwhile, Bambusa Therapeutics has announced the dosing of its first healthy volunteers as part of a Phase I trial on its half-life extended bispecific antibody therapy for AD.



