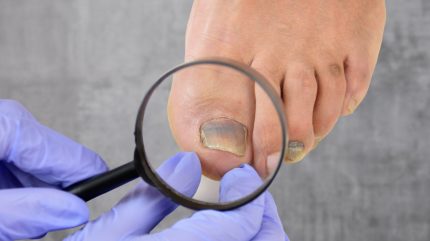
Almirall has completed the decentralised European regulatory procedure for efinaconazole, a triazole antifungal substance with the trade name Jublia, to treat onychomycosis.
This is a significant step in enhancing Almirall’s medical dermatology portfolio in Europe and the procedure is the last step before European countries can issue national marketing authorisations.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Onychomycosis, a fungal infection impacting an estimated 5.5% of the world population, accounts for half of all nail disorder consultations. It is a notoriously tenacious condition, often resistant to treatments.
Efinaconazole’s low binding affinity to keratin, key in nail composition, enables it to penetrate nails effectively.
In May 2022, Almirall initiated the regulatory submission process for efinaconazole in Germany and Italy, utilising the European decentralised procedure.
Germany’s regulatory authority acted as the reference member state.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAlmirall research and development, chief scientific officer and executive vice-president Dr Karl Ziegelbauer stated: “The completion of the decentralised regulatory procedure for efinaconazole in Europe enables European countries to approve this advanced treatment option to support people with onychomycosis.
“Efinaconazole as a topical treatment is a valuable addition to our expanding onychomycosis portfolio and we are looking forward to being able to offer it to European dermatologists and their patients in the near future.”
In July 2021, the company obtained exclusive development and marketing rights for efinaconazole in Europe from Kaken Pharmaceutical.
Kaken received an upfront cash payment from Almirall, along with milestone payments and royalties based on net sales of the product, which is currently available in the US, Canada, Korea, Hong Kong and Macau. It has been available in Japan under the trade name Clenafin since 2014.
Almirall is currently collaborating with national regulatory authorities, with authorisations anticipated in the first half of 2025.
In February 2024, Almirall entered a strategic collaboration with Microsoft for drug development in dermatology.


