The US Food and Drug Administration (FDA) has sent numerous warning letters to compounding pharmacies for illegally selling semaglutide and tirzepatide, or producing adulterated or misbranded versions. The FDA told the Washington Post that between August 2021 and mid-July 2024, it reviewed 288 reports of patients having bad reactions to compounded semaglutide and 108 such reports for tirzepatide. Some patients required hospitalisation.
Although GLP-1 agonist shortages have been improving recently, manufacturers are still struggling to keep up with high demand for these weight-loss drugs. Some patients are turning to compounding pharmacies when they cannot access the branded version of these drugs due to a shortage or other barriers such as cost and insurance coverage. However, the FDA, Eli Lilly (Indianapolis, IN, US), and Novo Nordisk (Bagsvaerd, Denmark) have all expressed quality and safety concerns with compounded weight loss drugs. The organisations found that some compounded versions of semaglutide and tirzepatide were counterfeit, contained too little, too much or no active ingredient at all, or contained the wrong or harmful ingredients.
Compounding is a well-established practice that allows for the customisation of dosage, formulation and delivery method to meet the specific needs of individual patients, such as for patients with an allergy to one of the inactive ingredients in the approved drug or for a patient who has trouble swallowing a tablet or capsule and needs medicine in a liquid form that is not otherwise available. In certain situations, compounding can be cheaper than purchasing FDA-approved medications, especially for patients who require specialised formulations or dosages. FDA-approved drugs can legally be compounded under certain conditions, though not if patented. The same is true for unapproved drugs. The FDA does allow compounders to make their own version if a drug is in shortage. In May 2023, Ozempic and Wegovy were both listed on the FDA’s drug shortages list, although shortages have lessened since then.
Warnings of adverse effects
The FDA recognises the substantial consumer interest in using compounded semaglutide products. However, compounded drugs pose a higher risk to patients than FDA-approved drugs because they do not undergo pre-market review for safety, effectiveness or quality. In July 2023, the FDA issued a warning after receiving several reports of adverse effects from people who used compounded semaglutide. It had received reports of dosing errors related to these compounded products. Some patients even sought medical attention or required hospitalisation after the medication error.
In August 2024, Endpoints News reported that Novo had filed 11 new lawsuits against medical spas, weight loss clinics and telehealth companies advertising compounded versions of its semaglutide products for diabetes and weight loss. The lawsuits were filed in California, Florida, Michigan, New Jersey, Oklahoma, Pennsylvania and Washington. The company has now filed a total of 34 legal actions against compounders, six of which have resulted in permanent injunctions.
Risk of bacterial contamination
Lilly stated that it had discovered compounded drugs advertised as tirzepatide with safety, sterility and efficacy problems. Some contained bacteria or high impurity levels, were differently coloured (pink, rather than colourless), or had a completely different chemical structure from Lilly’s FDA-approved medicines. In at least one instance, the product was nothing more than sugar alcohol.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataDan Skovronsky, chief scientific and medical officer of Eli Lilly, discussed the compounding problem in an earnings call on 8 August 2024, in the second financial quarter of the year. He stated: “We often are able to secure samples from these kinds of compounding labs and analyse them in our own labs. And what we found for the most part, in most instances, is this isn’t [our] kind of tirzepatide at all. Our drug is not available to compounders, rather they’re purchasing either other chemicals entirely, which we often find, or make [products] of tirzepatide that [are] often full of impurities [and] sometimes contaminated by bacteria. This is a safety risk to patients that we take seriously and try to think we can make patients aware of the potential dangers here so that we can help.”
On 21 May 2024, the Australian government announced a complete ban on compounded tirzepatide and other compounded diabetes and weight loss medicines due to “increasing reports of patients coming to harm from [compounded versions] including the hospitalisation of a patient in Australia due to a serious adverse event.”
Misbranded, adulterated and illegal sales
Skovronsky also noted that the number of diseases that could be treated with incretins (the category of hormones that includes GLP-1) is likely to rise, as will the number of oral versions, leading to increased demand, shortages and compounding. Incretin-based treatments reduce post-meal blood sugars. In the future, incretins could treat more disease indications, which will increase overall demand, shortages and the amount of future compounding for these drugs.
Scott Gottlieb, the former commissioner of the FDA, stated that “the problem with many compounded versions of GLP-1s is they’re a ‘salt’ formulation of the drug and this is likely to be more soluble and not provide the extended release of the weekly, brand versions. So they won’t deliver the expected benefit.”
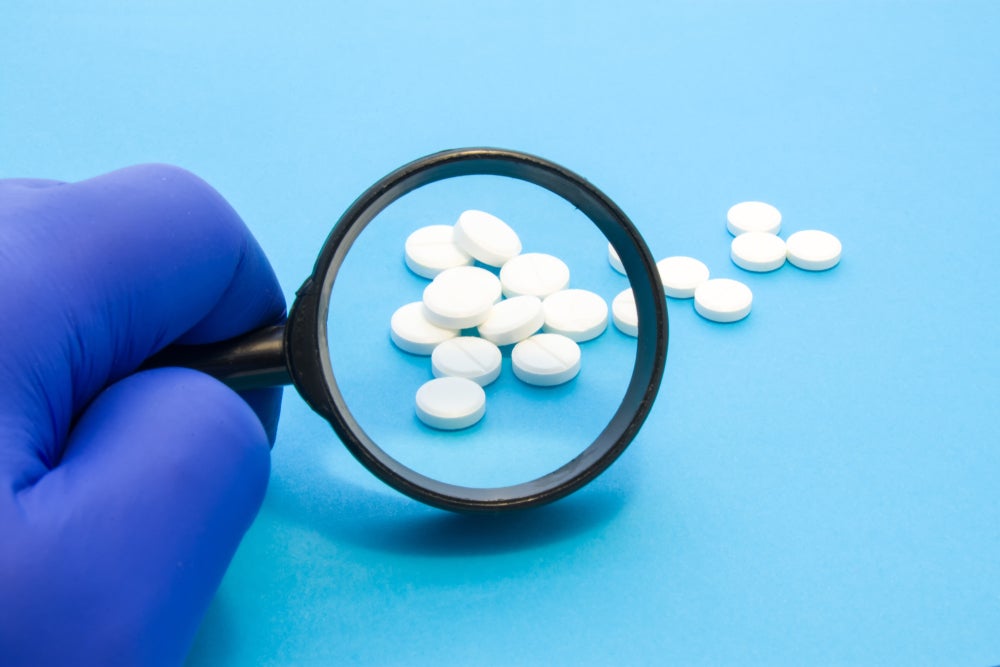
Since late 2023, the FDA has issued 10 warning letters regarding misbranded, adulterated or illegal sales of semaglutide and tirzepatide to the following companies: US Chem Labs (Miami, FL, US), Synthetix Inc. DBA Helix Chemical Supply (Bronx, NY, US) and AnuMed International, LLC (Phoenix, AZ, US), and to the websites gorillahealing, semaspace, Ozempen and dashpct. The websites for all these online retailers are currently unreachable. None of these companies’ facilities are registered as human drug compounding outsourcing facilities under section 503B of the Federal Food, Drug and Cosmetic Act.
The FDA has also designated 503B compounding pharmacies as manufacturing pharmacies that can produce large batches of medications without patient-specific prescriptions. They sell and directly distribute ready and reliable sterile medication to healthcare facilities. Compared to 503A pharmacies, facilities under 503B must follow more stringent manufacturing standards and are subject to FDA inspection.