Selkirk Pharma, a pharmaceutical contract manufacturer based in the US, has built a new sterile fill/finish manufacturing facility in Spokane, Washington. The facility, which has received aseptic process simulation (APS) certification, will significantly increase the capacity for commercial and clinical trial drug manufacturing.
The facility is the first building of three planned for Selkirk Pharma’s campus at the Pacific Northwest Technology Park.
Initial funding for the project was approximately $90m. A consortium of local healthcare providers and business owners have funded the project, with additional financing from the Embree Capital Markets Group.
Selkirk Pharma, along with its partners, invested an additional $60m into the facility. The investment facilitated the addition of new production lines and enhanced the facility’s capabilities.
Selkirk’s future expansion plans include the construction of a second manufacturing facility on an adjacent eight-acre (3.2-hectare [ha]) plot and a third facility on a 10.7-acre (4.3ha) site. Additionally, provisions have been made for grey shell space to accommodate two more filling lines.
Location
The facility is located at 9110 W Granite Avenue in Spokane, within Vandervert’s Pacific Northwest Technology Park.
The Vandervert’s Pacific Northwest Technology Park is a growing centre in the West Plains district of Spokane, close to Spokane International Airport which is less than half a mile away, facilitating efficient operations.
Selkirk Pharma sterile manufacturing facility details
Selkirk’s Plant 1 is built on a 115,000ft² (10,683m² ) area. It is purpose-built for contract manufacturing with a unidirectional flow for personnel, products and components to prevent cross-contamination and enhance manufacturing efficiency.
The facility is designed to manufacture sterile injectables, biologics and small molecules, aligning with Annex 1 compliance standards, which are crucial for clients targeting European and US markets. Selkirk Pharma’s facility incorporates new technology designed to be Annex 1 compliant.
Its design facilitates swift and adaptable expansion. The initial construction encompasses specialised compounding and filling areas within the Line 1 manufacturing zone. Provision has been made for two extra filling lines, complete with the necessary utilities and support infrastructure, allowing for seamless augmentation of manufacturing capacity and the incorporation of fresh capabilities as needed.
The facility includes 15,000ft² current good practice validated (cGMP), temperature-controlled space to handle all types of drug product logistics requirements, administration areas, laboratories, AES cleanroom production areas, mechanical spaces, warehousing and shipping spaces to support pharmaceutical drug production and distribution.
Selkirk Pharma sterile manufacturing facility design details
The facility includes a two-story main structure with a two-story entry lobby wing, and a single-storey high bay warehouse and loading dock wing.
The building’s exterior uses insulated metal panels and precast insulated concrete panels, serving as a weather barrier, thermal envelope and exterior finish. The use of the insulated panels allows for open wall cavities for electrical and communication conduits while maintaining thermal performance.
The facility features a single entry point for all staff, with secure access to clean areas. On the first floor, the facility houses the lobby, restrooms, offices, laboratories, pharmaceutical processing areas, equipment and product storage and shipping space. The interstitial/mezzanine level is dedicated to record storage and provides walkable access. It has dedicated compounding rooms for each flex filling line, and a full-height mechanical mezzanine.
A staff dining and community space on the second floor overlooks the grand lobby and can be used for company-wide meetings and presentations. It also includes additional office space and spaces for mechanical and electrical equipment. Flex space has also been included in the design to accommodate future expansion.
Additional features of the facility
The site includes ample parking, bicycle racks, a walking path, interior sidewalks, a plaza space and landscaping. Additionally, the facility is equipped to accommodate large truck access, loading docks, a mechanical and electrical yard and designated areas for trash and recycling within a secure back-of-house area.
The production core of the facility is a manufactured modular construction that includes unclassified laboratory spaces, as well as Controlled Not Classified ISO8 and ISO5 cleanroom spaces.
The fire protection system includes a comprehensive hazard analysis, preliminary sprinkler routing, hydraulic calculations and the determination of fire pump requirements, all made to the specific storage configurations of the user.
Technology details
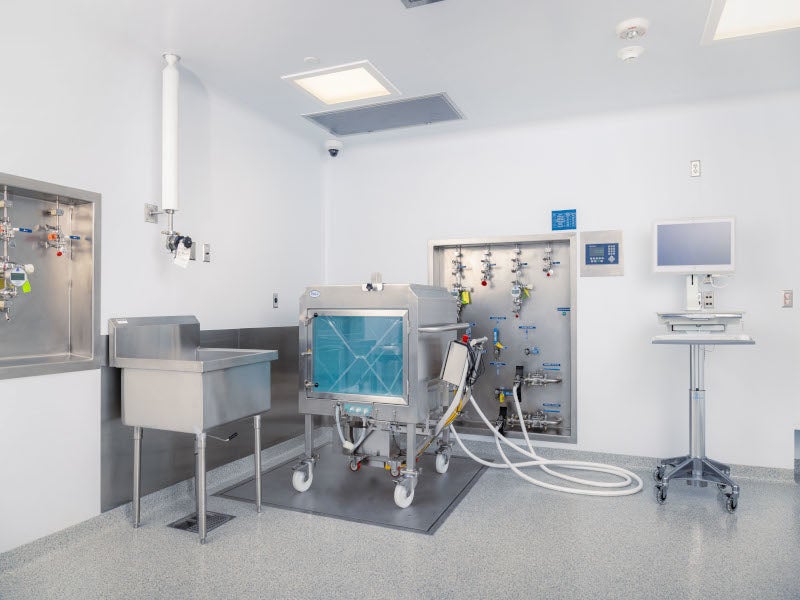
The facility utilises advanced sterile fill-finish capabilities with isolate-based filling technology.
It uses advanced technology, including single-use systems, SKAN isolator technology, and Bausch and Strobel VarioSys® advanced dose-filling systems to ensure high-quality yields for valuable biologics.
The Bausch and Strobel VarioSys filler, integrated with a vial rinser and depyrogenation tunnel, is fast, versatile and efficient, with minimal line losses and high container yields. The system is capable of handling bulk vial filling of 2ml to 30ml volumes at a rate of up to 3,600 vials per hour. Its efficacy is witnessed in a recovery rate of +99%, with minimal line loss and a filled container yield of greater than 99.8%.
The facility is also equipped with full manual quality control and automatic quality control capabilities, bulk packaging and a 100% visual manual inspection process. In-line non-destructive weight checks are also conducted, and the vials are prepared as ready-to-sterilise components, ensuring that the final product meets the highest standards of quality and safety.
The VarioSys technology is specifically designed for vial-based small-scale production runs, providing applications such as sterilisation, cleaning, precise dosing and container closure. The equipment includes a fully automatic cleaning machine for vials and bottles (FAW 1005, Bausch and Strobel make), a sterilising tunnel (DHT 2431, Bausch and Strobel make), a rapid decontamination system (SARA) for material transfer from SKAN, an isolator (PSI-L) from SKAN, and a VarioSys module for filling and stoppering vials (KSF 5105) from Bausch and Strobel.
The SKAN Isolator is the highest standard for aseptic filling, which brings state-of-the-art safety and efficiency to the filling operations. The system accommodates ready-to-use stoppers and over seals and the SKANFog system for rapid and thorough sterilisation of the entire filling system, ensuring the maximum level of protection for the container filling and sealing processes.
Selkirk Pharma sterile manufacturing facility services and capabilities
The facility offers real-time quality assurance review capabilities, automated data recording and fully integrated systems for inventory, engineering and quality management. It also provides a suite of testing services, including microbiological, sterility, bioburden and endotoxin tests, alongside the validation of analytical testing procedures.
Selkirk offers a comprehensive range of services, including aseptic fill and finish, analytical testing, manual product inspection and logistics and supply chain support.
The facility’s capabilities offer flexible batch sizes for clinical and commercial use, with options for single-use or stainless-steel tanks and dedicated formulation suites.
In terms of analytical support, the facility provides a full spectrum of testing, including microbiological assessments, high-performance liquid chromatography, ultra-performance liquid chromatography, gas chromatography and particulate matter testing. It also conducts International Council for Harmonisation stability testing and offers extensive resources for method transfer and validation.
Contractors involved
The Embree Development Group (EDG), a real estate company based in the US, was appointed by Selkirk Pharma as the development partner for the facility.
In the initial stages of the project, Sandis, a company specialising in engineering, surveying and planning based in the US, was contracted as a consultant. Collaborating with EDG and Vandervert Developments, Sandis played a pivotal role in securing the site for the new facility.
Sandis’ services included land consultation, analysis of boundaries and easements, boundary surveying, and the provision of an ALTA/NSPS land title survey. Additionally, Sandis was responsible for preparing and obtaining approval for an amended binding site plan from the city of Spokane, sanctioned by the County Auditor.
Coffman Engineers, an engineering consultancy company based in the US, was selected to provide mechanical, electrical, fire protection and civil engineering services for the facility.
DCI Engineers, a client-focused structural and civil engineering service provider based in the US, provided structural engineering for the project.
Bernardo Wills Architectures, a planning and design service company based in the US, provided full architecture, landscape architecture and interior design services, including a full fixtures and furniture package for the facility.
Bausch & Strobel, a pharmaceutical equipment manufacturing company based in Germany, was chosen to supply the VarioSys equipment.
SKAN, a company providing aseptic technology based in Switzerland, was contracted to provide a SKAN Isolator.