BioXcel Therapeutics. has filed a patent for methods of administering high doses of dexmedetomidine to treat agitation in patients with neurodegenerative or neuropsychiatric diseases without inducing sedation. The patent claim includes a range of canceled claims. GlobalData’s report on BioXcel Therapeutics gives a 360-degree view of the company including its patenting strategy. Buy the report here.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
According to GlobalData’s company profile on BioXcel Therapeutics, Cancer treatment biomarkers was a key innovation area identified from patents. BioXcel Therapeutics's grant share as of January 2024 was 15%. Grant share is based on the ratio of number of grants to total number of patents.
High dose dexmedetomidine for agitation in neurodegenerative diseases
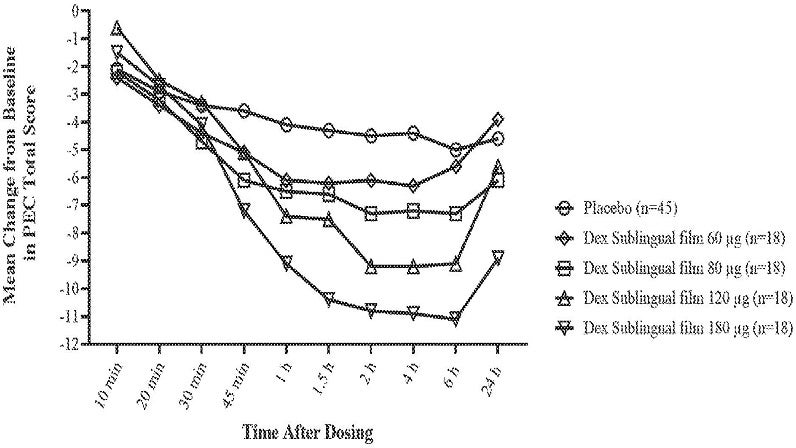
A patent application (Publication Number: US20240024288A1) discloses a method for treating agitation in individuals with schizophrenia or bipolar disorder by administering dexmedetomidine or its salt within a specific dosage range. The method involves achieving a total exposure of dexmedetomidine in the plasma within a defined range, resulting in the reduction of agitation symptoms. The dosage administered ranges from about 120 µg to 240 µg, with specific parameters for plasma concentration and maximum concentration levels outlined for effectiveness.
Furthermore, the method includes various administration routes such as oromucosal, oral, intranasal, or parenteral, with specific emphasis on sublingual and buccal administration. The patent also details the formulation of dexmedetomidine in the form of a film for sublingual administration, containing specific ingredients in defined proportions. The method also allows for a second dose to be administered within a specified time frame to maintain the reduction in agitation, as measured by standardized scales. Overall, the patent application provides a detailed and specific method for effectively treating agitation in individuals with schizophrenia or bipolar disorder using dexmedetomidine, highlighting the importance of dosage, administration route, and monitoring parameters for successful outcomes.
To know more about GlobalData’s detailed insights on BioXcel Therapeutics, buy the report here.
Data Insights
From

The gold standard of business intelligence.
Blending expert knowledge with cutting-edge technology, GlobalData’s unrivalled proprietary data will enable you to decode what’s happening in your market. You can make better informed decisions and gain a future-proof advantage over your competitors.



