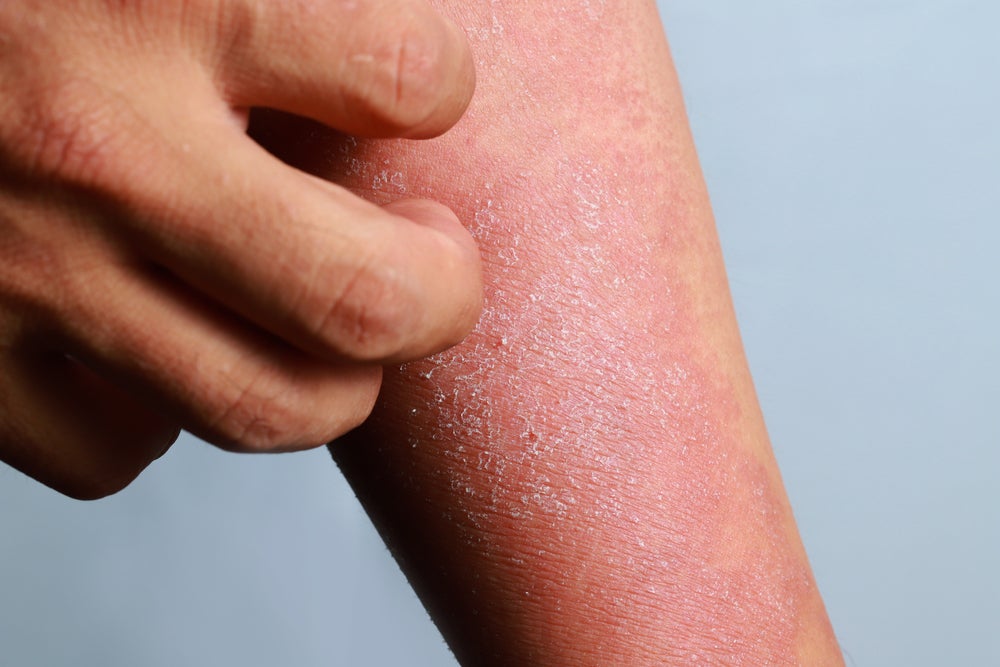
The European Commission (EC) is expected to approve Almirall’s Ebglyss (lebrikizumab) for atopic dermatitis after its Committee for Medicinal Products for Human Use (CHMP) issued a positive opinion for the treatment’s marketing authorisation.
The therapy is indicated for adult and adolescent patients with moderate-to-severe forms of the disease. Almirall, who has licence rights to develop and commercialise the drug in Europe for dermatology indications, says the therapy could be approved within two months.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Eli Lilly has rights for the drug in the rest of the world, including the US, where the drug was granted fast track designation by the US Food and Drug Administration (FDA) in 2019.
Ebglyss works by binding to the interleukin-13 protein and inhibiting its effects, which include driving the type-2 inflammatory loop in the skin. This type of inflammation leads to many of the features of atopic dermatitis, including skin irritation and redness.
The CHMP issued a positive opinion based on three pivotal Phase III studies. The ADvocate 1 and ADvocate 2 trials tested Ebglyss as a monotherapy, and the Adhere trial examined the drug in combination with topical corticosteroids.
In May, Eli Lilly unveiled data from randomised trials showing that up to 73% of patients receiving the treatment had enhanced or cleared skin on their face or hands at 16 weeks. Additionally, 80% of patients who achieved 75% in lesion extent and severity (EASI-75) by week 16, maintained the improvement after a year.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataProfessor Alan Irvine, a dermatologist in Children’s Health Ireland and St James’s Hospital, Dublin, Ireland, said: “Lebrikizumab’s targeted mechanism of action inhibits IL-13 signalling. In clinical trials, it helped patients control their disease and maintain those results long-term, over 52 weeks.
“Additionally, its monthly maintenance dosing regimen offers convenience and flexibility, benefiting both patients and healthcare providers.”
Sanofi and Regeneron’s Dupixent (dupilumab), an IL-13 and IL-14 inhibitor, currently dominate the treatment space. According to GlobalData, the drug is predicted to encounter sales of $19.7bn by 2029.
GlobalData is the parent company of Pharmaceutical Technology.




