Spectrum Pharmaceuticals has been granted a patent for a method of preventing or treating neutropenia, a condition characterized by compromised white blood cell production. The method involves administering a protein complex, consisting of a modified human granulocyte-colony stimulating factor (hG-CSF) linked to an immunoglobulin Fc region via a non-peptidyl polymer, on the same day as a chemotherapy regimen. The modified hG-CSF has specific amino acid substitutions. GlobalData’s report on Spectrum Pharmaceuticals gives a 360-degree view of the company including its patenting strategy. Buy the report here.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
According to GlobalData’s company profile on Spectrum Pharmaceuticals, Cancer treatment biomarkers was a key innovation area identified from patents. Spectrum Pharmaceuticals's grant share as of June 2023 was 1%. Grant share is based on the ratio of number of grants to total number of patents.
Method of treating neutropenia with modified hg-csf protein complex
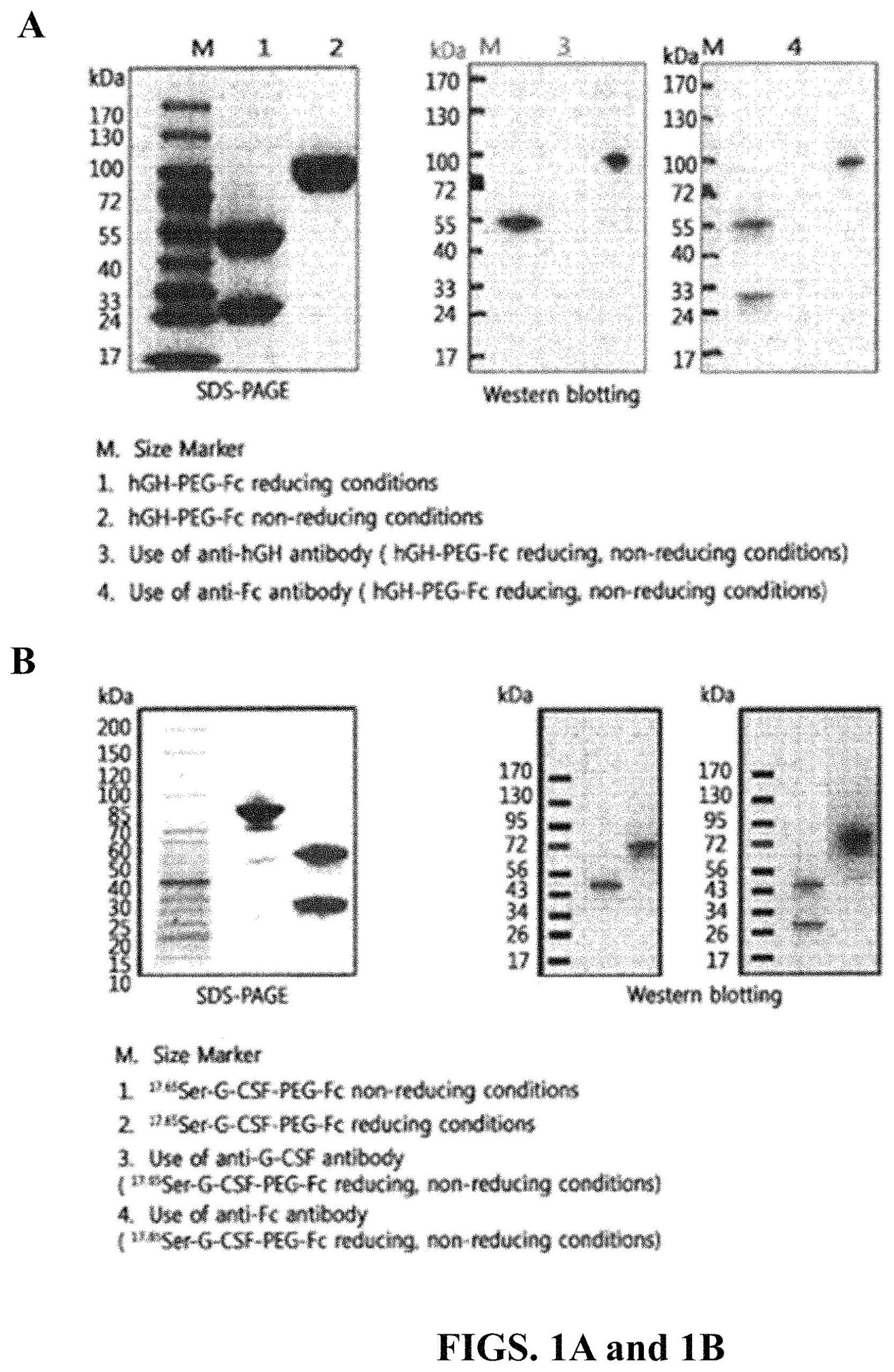
A recently granted patent (Publication Number: US11684655B2) describes a method for treating a condition characterized by compromised white blood cell production in a subject. The method involves administering a chemotherapeutic regimen followed by a protein complex comprising a modified human granulocyte-colony stimulating factor (hG-CSF) covalently linked to an immunoglobulin Fc region via a non-peptidyl polymer. The protein complex is administered on the same day as the chemotherapeutic regimen.
The condition that can be treated using this method includes reduced hematopoietic function, reduced immune function, reduced neutrophil count, reduced neutrophil mobilization, mobilization of peripheral blood progenitor cells, sepsis, severe chronic neutropenia, bone marrow transplants, infectious diseases, leucopenia, thrombocytopenia, anemia, enhancing engraftment of bone marrow during transplantation, enhancing bone marrow recovery in treatment of radiation, chemical or chemotherapeutic induced bone marrow aplasia or myelosuppression, and acquired immune deficiency syndrome.
The method can be used specifically for treating neutropenia, particularly febrile neutropenia, which is a result of chemotherapy, radiation therapy, or idiopathic thrombocytopenic purpura. The protein complex can be administered after the subject is treated with adjuvant or neoadjuvant chemotherapy, and the second dose of the protein complex can be administered between 15 and 25 days after the first dose.
The therapeutically effective amount of the protein complex is between about 5 µg/kg and about 200 µg/kg, with specific unit dosage forms ranging from about 9 µg/kg to about 200 µg/kg. The protein complex can be administered within various timeframes after the completion of the chemotherapeutic regimen, ranging from 30 minutes to 12 hours.
The protein complex is formed by covalently linking the modified hG-CSF to the immunoglobulin Fc region via a non-peptidyl polymer. The non-peptidyl polymer can have reactive groups such as aldehyde, maleimide, or succinimide derivatives at both ends. The immunoglobulin Fc region can be aglycosylated and consist of one to four domains, including CH1, CH2, CH3, and CH4 domains, and may also include a hinge region. The immunoglobulin Fc region can be derived from IgG, IgA, IgD, IgE, or IgM.
Overall, this patent describes a method for treating conditions characterized by compromised white blood cell production using a specific protein complex administered after a chemotherapeutic regimen. The method offers potential benefits for patients undergoing chemotherapy or radiation therapy, as well as those with various hematopoietic and immune-related conditions.
To know more about GlobalData’s detailed insights on Spectrum Pharmaceuticals, buy the report here.
Data Insights
From

The gold standard of business intelligence.
Blending expert knowledge with cutting-edge technology, GlobalData’s unrivalled proprietary data will enable you to decode what’s happening in your market. You can make better informed decisions and gain a future-proof advantage over your competitors.



