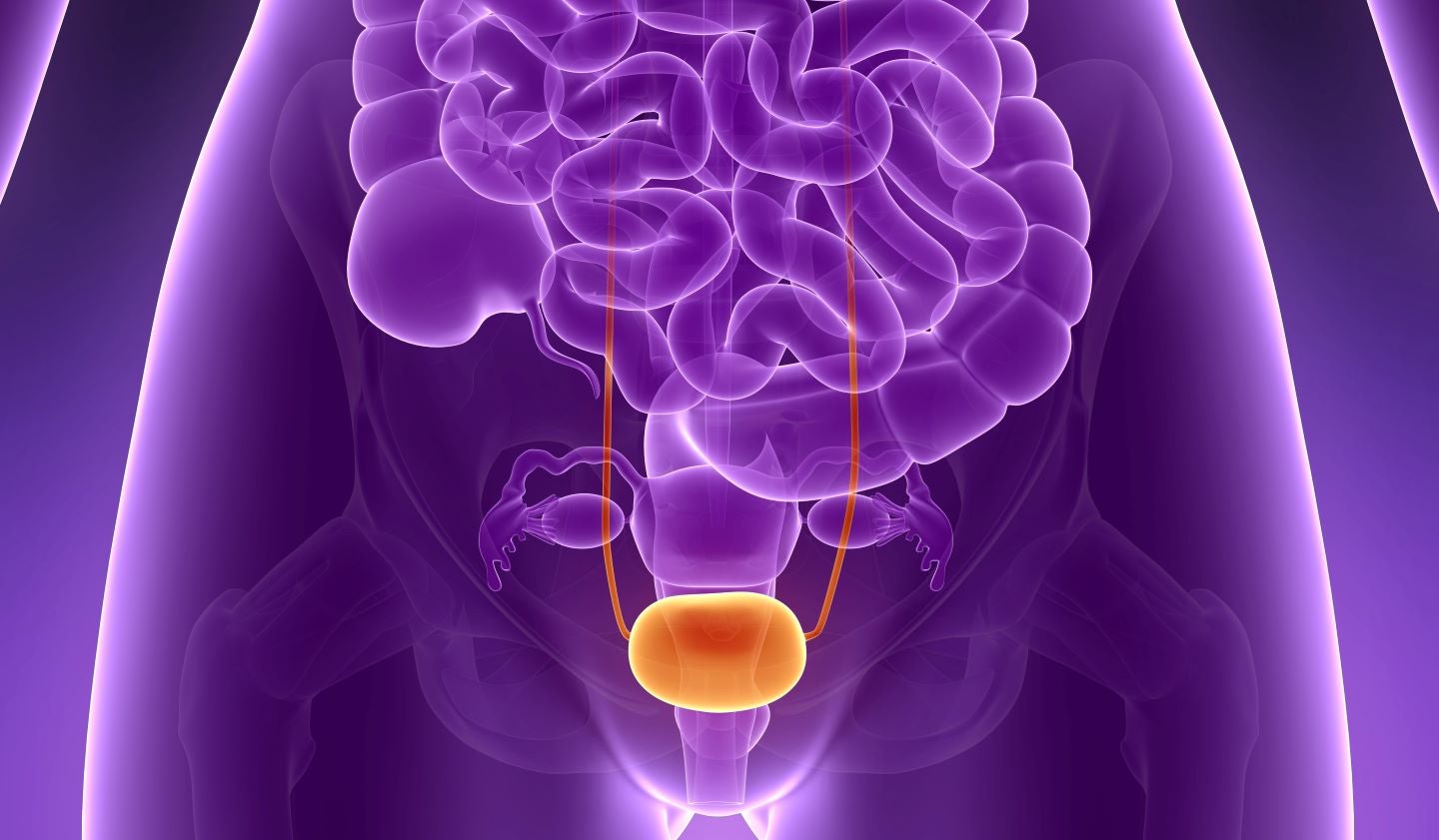
Oncolytic immunotherapy company CG Oncology has secured $105m in a crossover funding round to advance its clinical-stage bladder cancer programmes.
The financing round was led jointly by new investors Foresite Capital and TCGX. BVF Partners, Avidity Partners and Janus Henderson Investors joined them.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Current investors Acorn Bioventures, Decheng Capital, Ally Bridge Group, Malin Corporation, RA Capital Management and Longitude Capital also took part in the round.
CG Oncology plans to use the oversubscribed financing to progress clinical programmes in bladder cancer and obtain approval from the US Food and Drug Administration (FDA).
The programmes include the single-arm, Phase III BOND-003 monotherapy clinical trial of cretostimogene grenadenorepvec. This could potentially treat patients with high-risk non-muscle invasive bladder cancer (NMIBC) not responsive to bacillus Calmette-Guerin (BCG).
Cretostimogene grenadenorepvec is an oncolytic immunotherapy agent administered intravesically. It is being evaluated in a Phase II trial along with Keytruda (pembrolizumab) for the same indication.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe agent is also being analysed along with Opdivo (nivolumab) for the treatment of other bladder cancer types.
CG Oncology CEO Arthur Kuan stated: “We are excited to welcome leading life science investors who share our vision of developing cutting-edge therapeutics addressing unmet medical needs in bladder cancer.
“Our lead asset, cretostimogene grenadenorepvec, continues to make significant clinical progress in bladder cancer in both monotherapy and combination studies. We are encouraged to see our treatments get closer to being available to bladder cancer patients worldwide.”




