Shin Poong Pharm has filed a patent for the use of pyronaridine or artemisinin in preventing or treating RNA viral infectious diseases, specifically COVID-19. GlobalData’s report on Shin Poong Pharm gives a 360-degree view of the company including its patenting strategy. Buy the report here.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
According to GlobalData’s company profile on Shin Poong Pharm, cancer treatment biomarkers was a key innovation area identified from patents. Shin Poong Pharm's grant share as of June 2023 was 1%. Grant share is based on the ratio of number of grants to total number of patents.
Use of pyronaridine or artemisinin for treating covid-19
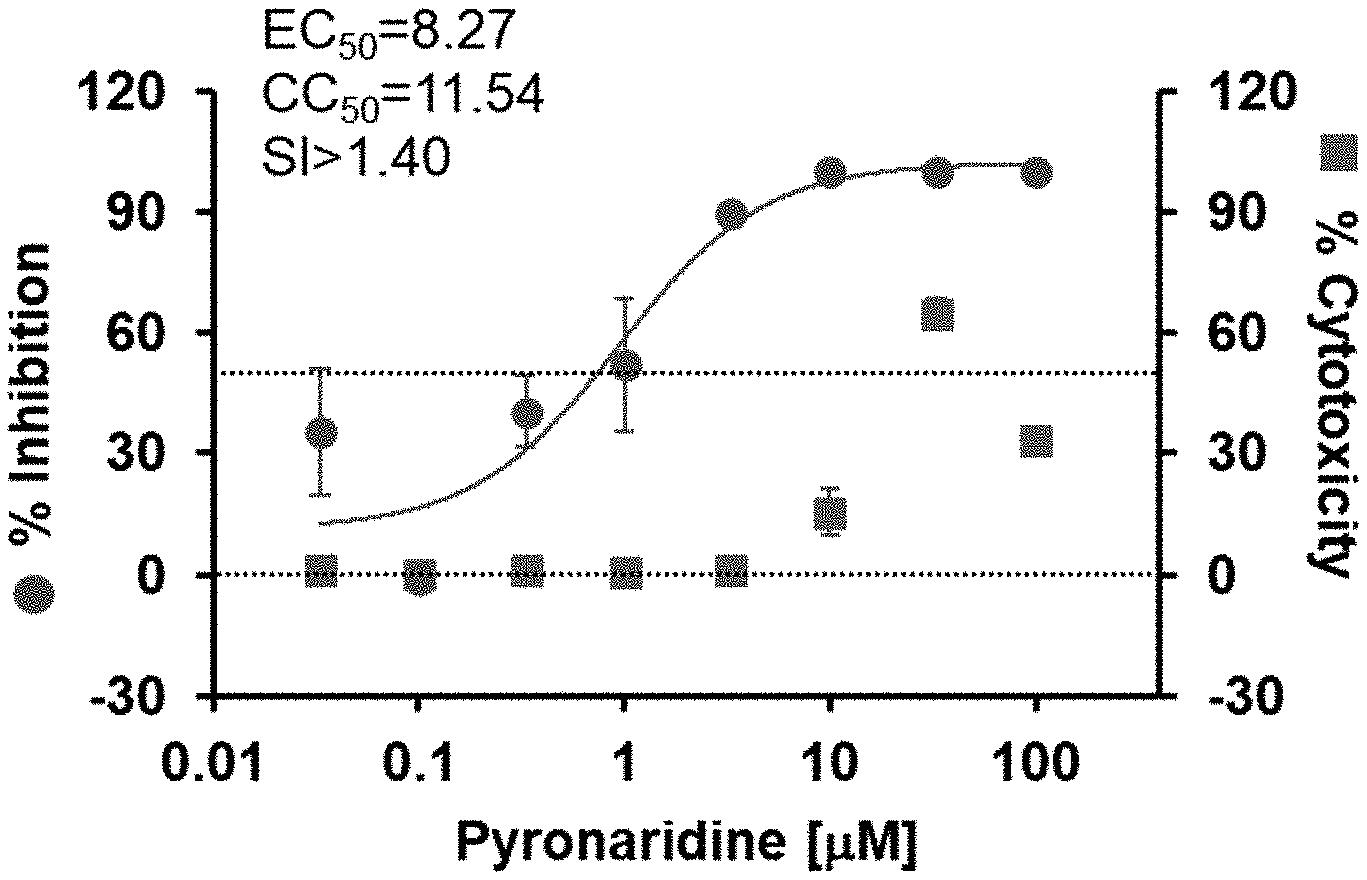
A recently filed patent (Publication Number: US20230132036A1) describes a method for treating epidemic RNA virus infections. The method involves administering either pyronaridine or artemisinin, along with their respective pharmaceutically acceptable salts, to a patient in need.
Claim 33 states that the method involves administering a therapeutically effective amount of pyronaridine or artemisinin to a patient. Claim 34 expands on this by suggesting the administration of both pyronaridine and artemisinin to the patient.
The patent also specifies the use of various pharmaceutically acceptable salts of pyronaridine, such as phosphate, sulfate, hydrochloride, acetate, methanesulfonate, benzenesulfonate, toluenesulfonate, maleate, and fumarate. Claim 36 specifically mentions pyronaridine tetraphosphate as a preferred salt.
The weight ratio of pyronaridine or its salt to artemisinin or its derivative is discussed in claims 36, 37, and 38. The ratio can range from 10:1 to 1:10, with claim 38 specifying a ratio of 3:1.
The patent also mentions the use of artemisinin derivatives, such as dihydroartemisinin, artesunate, artemether, and arteether. Claim 40 specifically mentions artesunate as a preferred derivative.
Claims 41 and 42 suggest the administration of at least one other antiviral agent along with pyronaridine and artemisinin. These agents can include viral replication inhibitors, helicase inhibitors, viral protease inhibitors, and viral cell entry inhibitors. Examples of such agents mentioned in claim 44 are ribavirin, interferon, niclosamide, or a combination thereof.
The patent also specifies the epidemic RNA virus infections that can be treated using this method. These include Zika virus infection, Ebola virus infection, and respiratory diseases caused by novel influenza virus or coronavirus infections. Specifically, claim 46 mentions Severe Acute Respiratory Syndrome (SARS), Middle East Respiratory Syndrome (MERS), and Coronavirus Disease 2019 (COVID-19) as respiratory diseases caused by coronavirus infection. Claim 47 specifically mentions COVID-19 as a respiratory disease caused by coronavirus infection.
In summary, the patent describes a method for treating epidemic RNA virus infections by administering pyronaridine or artemisinin, along with their respective salts, to a patient in need. The method can also involve the administration of other antiviral agents and is applicable to various epidemic RNA virus infections, including COVID-19.
To know more about GlobalData’s detailed insights on Shin Poong Pharm, buy the report here.
Data Insights
From

The gold standard of business intelligence.
Blending expert knowledge with cutting-edge technology, GlobalData’s unrivalled proprietary data will enable you to decode what’s happening in your market. You can make better informed decisions and gain a future-proof advantage over your competitors.



