NorthX Biologics has acquired Valneva’s clinical trial manufacturing unit in Stockholm, Sweden.
The takeover comprises the transfer of a multi-purpose site with 30 staff, located in the Stockholm life sciences cluster.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
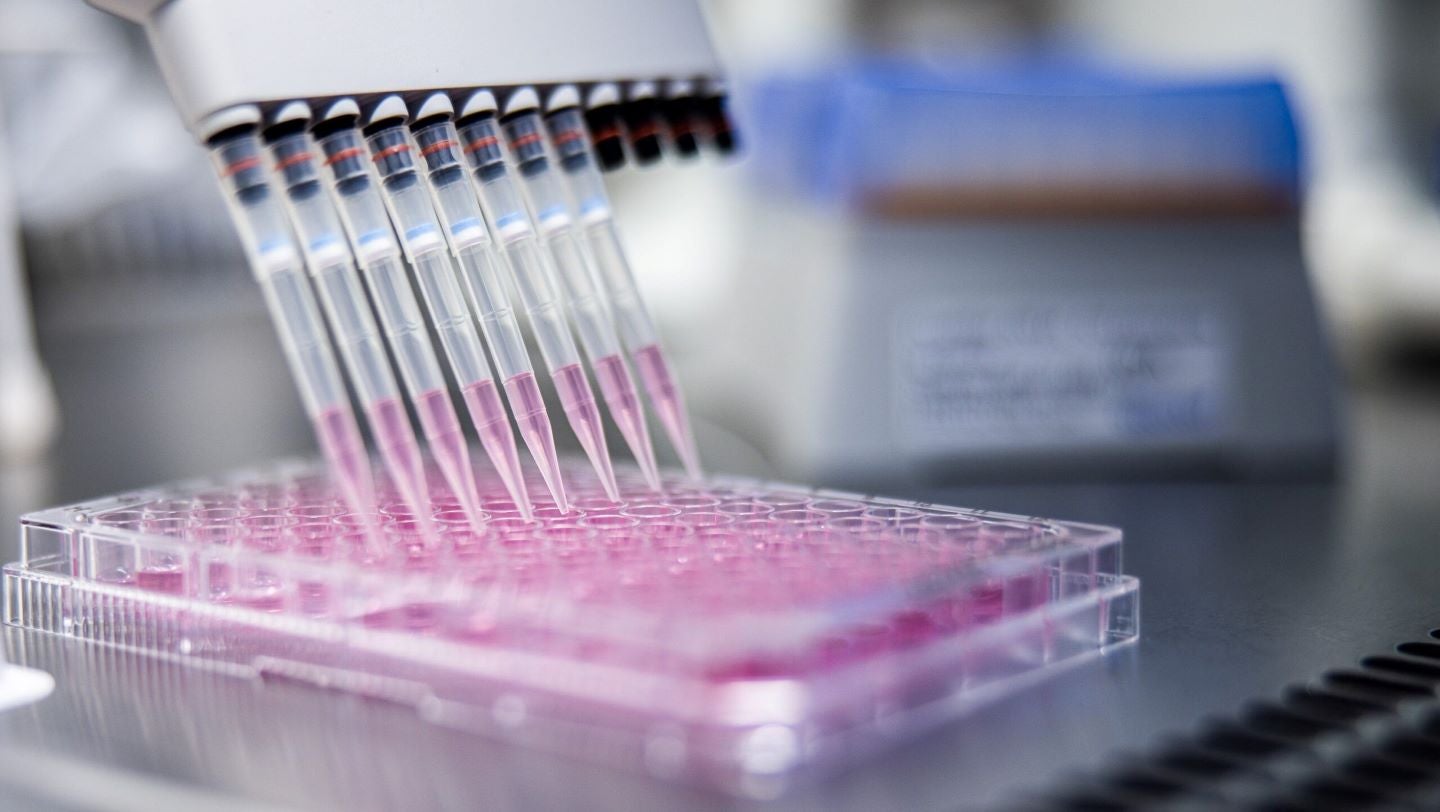
The staff and facility have a long history of experience in internal services, contractual development and production activities with external clients.
Their capabilities in mammalian expression systems and viral vectors complement the expertise of NorthX’s existing business of sophisticated microbial-based protein and plasmid deoxyribonucleic acid (DNA) production.
The acquired unit possesses expertise in process development, GMP manufacturing, ramp-up, quality assurance/release and control analytics. It can also handle biosafety level (BSL) 2/2+ and BSL 3 organisms.
This acquisition will support NorthX in bolstering its expertise and providing complete services to a global client base.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataValneva Sweden managing director Janet Hoogstraate will become part of NorthX following the deal’s closure.
NorthX Biologics chairman Thomas Eldered stated: “This strategic move marks a significant milestone in our growth journey and strengthens NorthX as Sweden’s innovation hub.
“We are now able to work with ATMPs and advanced biologics, including process development and manufacture for clinical trials and commercial requirements.”




