On 9 May, National Fentanyl Awareness Day was observed in recognition of the US opioid crisis. Accordingly, the development of non-opioid painkillers is experiencing a surge in activity and despite numerous novel targets receiving high levels of attention, cannabinoids have emerged as strong favourites to replace opioid-related medications.
The North American opioid epidemic highlights limitations in opioid use, such as the potential for drug abuse and overdose. Addiction affects an estimated three million US citizens and claimed over 100,000 lives in 2021 alone. Nevertheless, opioid-based painkillers remain the primary approach for pain management, creating a need for effective drugs with alternative targets. Currently, there are 546 painkillers in active development, with 458 targeting opioid-receptor alternatives, according to GlobalData’s Drugs Database. A promising example includes Vertex Pharmaceuticals’s VX-548, a sodium channel subunit blocker. In 2022, the FDA awarded VX-548 breakthrough therapy and fast-track designations for post-operative pain, and the drug is forecast to reach sales of $416m by 2029.
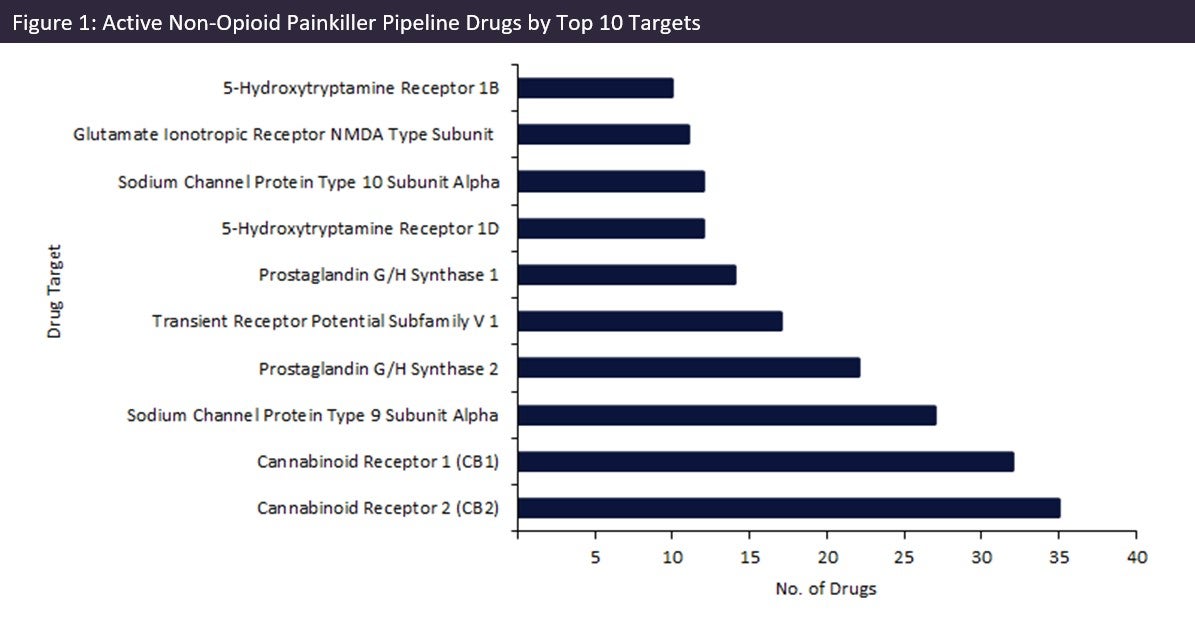
Of these 458 drugs, drugs targeting cannabinoid receptors (CB) are currently the two most popular targets, with 35 drugs in development for CB2, and 32 targeting CB1 (Figure 1, above), accounting for approximately 10% of pipeline drugs. These receptors interact with naturally occurring or synthetic cannabinoids to modulate a variety of physiological processes, including pain. CB1 is predominantly found in the central and peripheral nervous system, whereas CB2 is primarily located in immune cells. As drug targets, these receptors have demonstrated therapeutic value against different pain varieties. However, along with other painkiller alternatives, doubts have been cast over their efficacy.
Six cannabinoids have recently completed or are undergoing Phase II trials. A leading CB2 agonist, olorinab, displayed encouraging results when managing abdominal pain in irritable bowel syndrome (IBS) patients, but failed to meet the primary efficacy endpoints. In 2022, Pfizer acquired the drug, with the promise of accelerating clinical development via new, differentiated, and best-in-class approaches, enhancing optimism for further drug development. Currently, Jazz Pharmaceuticals’s nabiximols is the only cannabinoid approved for pain, receiving approval in Israel, in August 2010. However, 2017 saw the discontinuation of nabiximols from Phase III development in the US for cancer pain, due to lack of efficacy. Unfortunately, this is a recurring theme, with AstraZeneca discontinuing its non-opioid painkiller, AZD-1704, from Phase I development, for a similar reason.
Although efficacy concerns hinder the use of cannabinoids as a solution to the crisis, intensified research contributes to a better understanding of this domain, enabling a greater efficacious potential of future marketed treatments. The large number of pipeline cannabinoid painkillers suggests the future of pain medication may lie within this area, instilling hope for a future reduction in opioid manufacturing and prescriptions, and a potential route out of the crisis.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData