InDex Pharmaceuticals has entered a licence deal with Viatris Pharmaceutical Japan for the development and commercialisation of cobitolimod in Japan to treat ulcerative colitis (UC).
Under the terms of the deal, InDex will receive a $10m upfront payment from Viatris Japan for the exclusive commercialisation rights for cobitolimod in the country.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The company will also be entitled to receive up to $40m in additional payments linked to development and sales milestones.
The licensing agreement also requires Viatris Japan to pay up to double-digit percentage royalties based on the net sales of the product in Japan.
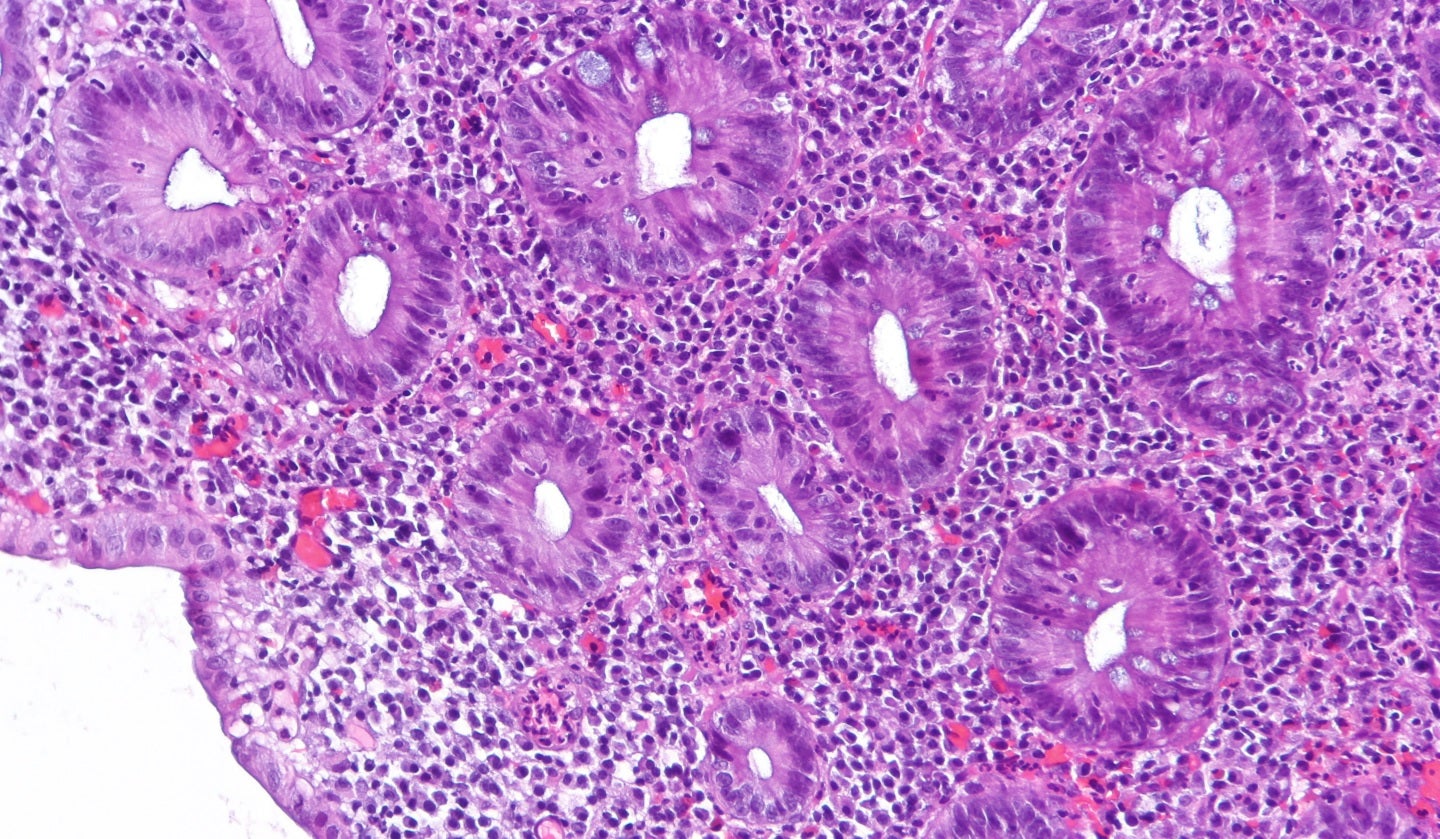
A first-in-class Toll-like receptor 9 (TLR9) agonist, cobitolimod is administered locally in the large intestine to offer a local anti‐inflammatory effect.
This can lead to mucosal healing and clinical symptom improvement in UC.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe therapy is currently being assessed in an international Phase III CONCLUDE programme to treat moderate to severe UC.
According to the agreement, InDex will continue to provide funding for all the development, including the completion of the Phase III CONCLUDE programme, a Japanese patients cohort in Induction Study 2, as well as a pharmacokinetic (PK) study in Japanese patients before filing.
InDex CEO Jenny Sundqvist said: “We are excited to start our collaboration with Viatris Japan with the common goal to register and commercialise cobitolimod for the Japanese market.
“Viatris Japan shares our view of how best to position cobitolimod for the treatment of moderate to severe ulcerative colitis in the Japanese market.
“Their experience and presence in the local gastrointestinal segment will ensure that we maximise our sales potential and bring the benefits of this innovative therapy to patients.”
Viatris Japan will be responsible for providing funding for all the regulatory and commercialisation activities, along with any further Japan-specific trials required in the country.




