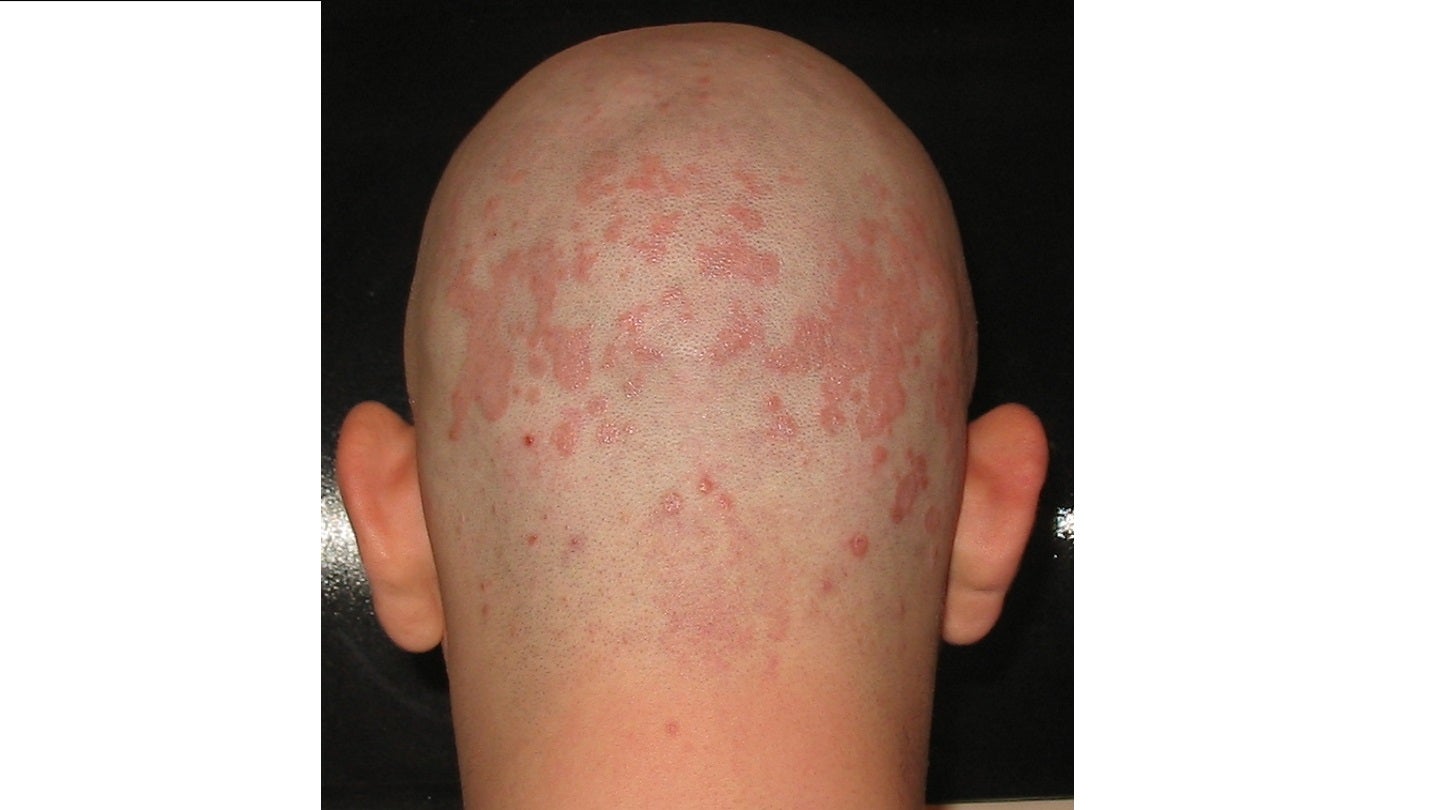
The US Food and Drug Administration (FDA) has accepted Arcutis Biotherapeutics’ new drug application (NDA) for roflumilast foam 0.3% to treat patients aged nine years and above with seborrheic dermatitis.
The regulator has set 16 December 2023 as a prescription drug user fee act (PDUFA) target action date for the decision on the application.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Roflumilast foam is an investigational, potent and selective phosphodiesterase type 4 (PDE4) inhibitor. The drug is taken once a day and is currently being developed for the treatment of inflammatory dermatoses, mainly in hair-bearing body areas such as the face, the trunk and the scalp.
Arcutis Biotherapeutics president and CEO Frank Watanabe stated: “The acceptance of the NDA for roflumilast foam marks a major milestone toward our goal of bringing a steroid-free, topical foam treatment option to market, addressing a significant unmet need for individuals living with seborrheic dermatitis.
“If approved, roflumilast foam would be the first topical drug with a new mechanism of action for this condition in over two decades, highlighting the unique formulation and deep dermatological expertise that Arcutis brings to immuno-dermatology.”
The NDA submission to the FDA was based on positive data obtained from the Phase II and pivotal Phase III trials of roflumilast foam.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe vehicle-[an inert medium in which a medicinally active agent is administered]controlled, parallel-group, pivotal double-blind Phase III study of roflumilast foam applied topically for the reduction of seborrheic dermatitis (STRATUM) trial was designed to assess the efficacy and safety of roflumilast foam 0.3% in patients with seborrheic dermatitis.
The findings showed that the trial met its primary endpoint, with a 79.5% investigator global assessment (IGA) success rate in patients treated with roflumilast foam compared to 58% in individuals treated with the vehicle at the end of week eight.
Roflumilast foam had a favourable safety profile and was also found to be well-tolerated.




