Bayer HealthCare has been given two separate orphan drug designations from the US Food and Drug Administration’s (FDA) Office of Orphan Products Development for its investigational oral drug riociguat, for treating pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH).
The US Orphan Drug Act grants special status for medications developed to treat and prevent rare medical conditions and diseases.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Bayer first submitted a new drug application (NDA) for riociguat in February 2013 for the treatment of PAH in order to improve exercise capacity and as a remedy against persistent or recurrent CTEPH.
Riociguat has been approved for use under the trade name Adempas by Health Canada for the treatment of inoperable, or persistent or recurrent CTEPH in adult patients.
This drug is being studied as a new method to treat different types of pulmonary hypertension (PH).
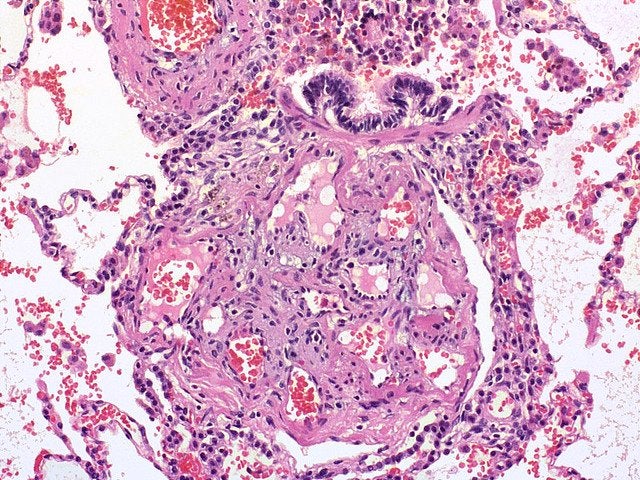
PAH is one of the five types of PH and is life-threatening because it causes the blood pressure in the pulmonary arteries to increase, which can result in heart failure.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataPAH is a rare disease, affecting an estimated 52 people per million worldwide.
CTEPH is also a life-threatening illness and a type of PH that causes blood clots in vessels, which gradually leads to high blood pressure in the arteries and a potential overload of the heart’s right side.
Bayer said despite the availability and benefits of several PAH therapies, outcomes for many patients remain poor, which indicates that new treatment options are needed.
If Riociguat is approved by the FDA, it would lead to the availability of a new class of pulmonary hypertension drugs in the US, the company added.
Image: A view of a plexiform lesion showing pulmonary hypertension. Photo: courtesy of Wikipedia.




