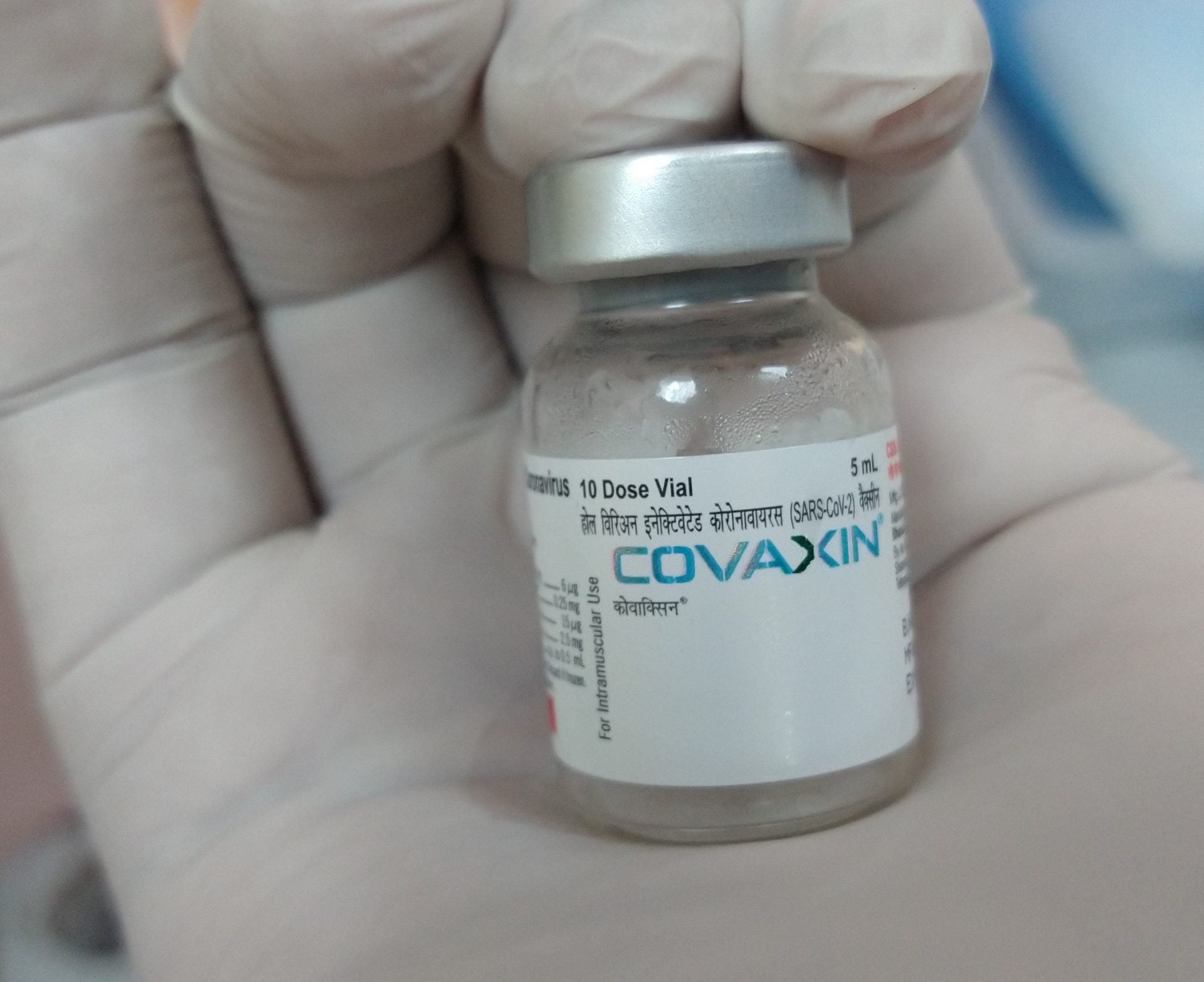
The Drug Controller General of India (DGCI) has granted Emergency Use Authorization (EUA) to Bharat Biotech’s Covid-19 vaccine, Covaxin, for use in children aged 12 to 18 years.
In October this year, an expert panel of Central Drugs Standard Control Organisation (CDSCO) recommended granting EUA to the vaccine for use in kids aged two to 18 years.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
With the latest approval, Covaxin has become the second shot to be cleared for usage in children in the country.
Earlier this month, ZyCoV-D became the first Covid-19 vaccine to be approved by the drug regulator for those aged 12 and above.
Initially, ZyCoV-D will be administered to adults in seven Indian states.
On 25 December, India also announced Covid vaccination for children aged from 15 to 18 years to commence from 3 January 2022.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn Phase III clinical trial, Covaxin demonstrated an efficacy of 77.8% against mild, moderate and severe Covid-19.
Separately, Japan’s Ministry of Health, Labor and Welfare (MHLW) has granted Special Approval for Emergency for Merck (MSD) and Ridgeback’s oral antiviral, molnupiravir, for Covid-19.
In a supply agreement signed last month, the Japanese government had agreed to procure nearly 1.6 million courses of antiviral to expand access to people.
Molnupiravir will be sold under the brand name Lagevrio in Japan.
The latest MHLW approval is based on favourable interim assessment data from the Phase III MOVe-OUT clinical trial of 800mg twice a day dose of molnupiravir in adult Covid-19 patients with mild-to-moderate disease.
Molnupiravir substantially lowered the hospitalisation and morality risk with only 7.3% treated with the oral drug being admitted to the hospital, while 14.1% in the placebo arm were admitted to hospital or died.
Furthermore, no cases of death were reported in the molnupiravir arm through day 29 as against eight in the placebo arm.
Merck Research Laboratories president Dr Dean Li said: “As a single oral medicine that can be taken at home, early treatment with molnupiravir significantly reduced the risk of hospitalisation or death in patients at high risk for progressing to severe Covid-19.
“We believe that molnupiravir will be a critical addition to the measures available to help curb the impact of Covid-19 on patients, healthcare systems and public health in Japan.”
Last week, molnupiravir obtained EUA from the US Food and Drug Administration for use in adults with mild to moderate Covid-19.




