Sanofi has signed a definitive agreement to acquire the entire share capital of Kiadis Pharma in a deal valued at approximately €308m ($359m).
Under the agreement, Sanofi will offer €5.45 per share to Kiadis Pharma, a clinical-stage company developing cell-based immunotherapy products.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
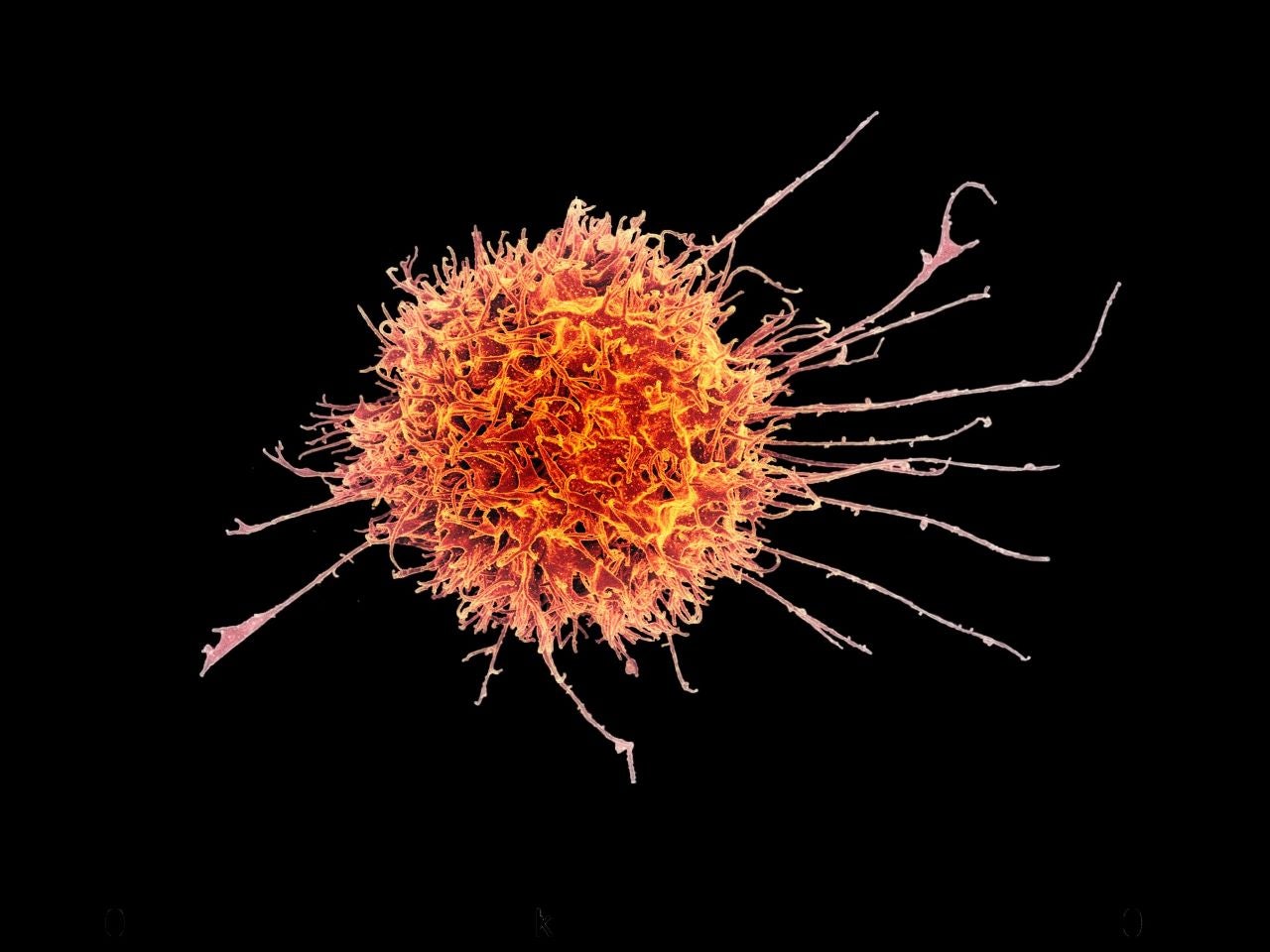
The acquisition will give Sanofi full control of next-generation natural killer (K-NK) cell platform and pipeline of cell-based cancer immune-therapeutics and infectious disease treatments.
The K-NK cell platform derives from allogeneic or ‘off-the-shelf’ NK cells from a healthy donor and these cells hunt for malignant cancer cells.
It can potentially make products rapidly and economically available for a broad range of indications.
Under the deal, Kiadis will develop NK cell-based medicines alone or with Sanofi’s existing platforms.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataFurthermore, the research, development and commercial expertise of Sanofi will be leveraged to advance Kiadis’ pipeline that includes NK cell-based medicines.
Sanofi Research & Development global head John Reed said: “We believe the Kiadis ‘off the shelf’ K-NK cell technology platform will have broad application against liquid and solid tumours, and create synergies with Sanofi’s emerging immuno-oncology pipeline, providing opportunities for us to pursue potential best-in-disease approaches.”
On completion of the public offer, Sanofi will provide the resources and capabilities needed for boosting the development of Kiadis’ programmes.
Kiadis’ pipeline of NK cell therapies includes K-NK002, K-NK003 and KNK-ID-101.
KNK-ID-101 is a programme analysing the properties of K-NK cells and their suitability to fight SARS-CoV-2. It will also analyse whether the cells can be used as a post-exposure pre-emptive therapy for Covid-19 in high-risk patients.
The company anticipates initiating a Phase I / IIa trial evaluating the use of K-NK cells to treat covid-19 patients with government grant funding.
In July, the company licensed Kiadis’ pre-clinical K-NK004 programme for potential combination for multiple myeloma.



