Vaccination is being considered as the only way the coronavirus pandemic can be controlled, although a highly effective vaccine is not expected to be available in less than a year.
More than 280 COVID-19 vaccines are in various phases of development across the world, according to GlobalData. Verdict has conducted a poll to assess which of the emerging COVID-19 vaccines are most promising.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
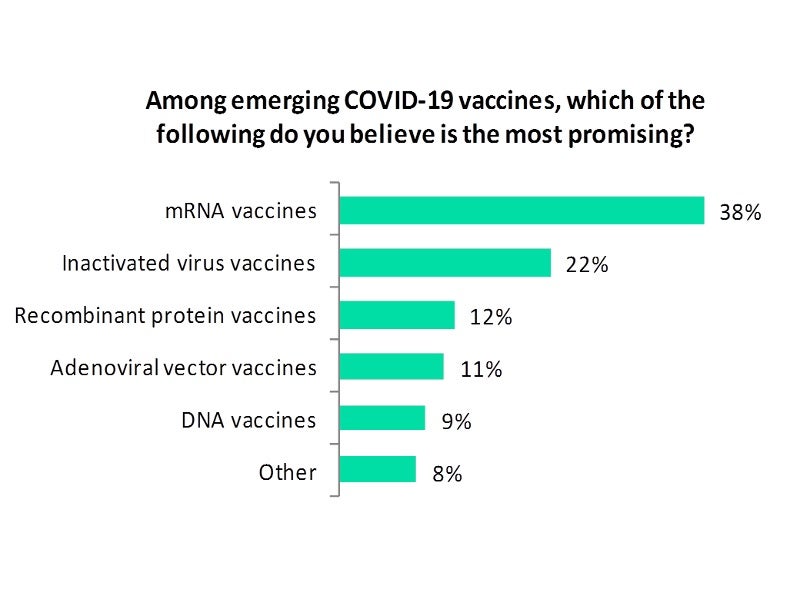
Analysis of the poll results shows that mRNA vaccines are being seen as the most promising, as opined by a majority of 38% of the respondents.
While 22% of the respondents felt that inactivated virus vaccines are the most promising among emerging vaccines, 12% felt that recombinant protein vaccines are the most promising.
Further, 11% of the respondents voted that adenoviral vector vaccines are the most promising followed by 9% who considered DNA vaccines as the most promising.
A minority 8% of the respondents opined that other types of vaccines are the most promising.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData
The analysis is based on 373 responses received between 08 July and 10 August from readers of Pharmaceutical Technology, a Verdict network site.
COVID-19 vaccine developments that have advanced
Scientists and pharmaceutical companies are following different approaches to developing vaccines against coronavirus. The vaccine being developed by Oxford University in partnership with AstraZeneca is the first to show efficacy against the coronavirus. It is an adenovirus vaccine vector designed to generate an immune response.
Another leading vaccine being developed by Johnson & Johnson/Janssen Pharmaceuticals is also an adenovirus vaccine vector, while those being developed by Merck, Sharpe & Dohme/International AIDS Vaccine Initiative are based on recombinant vesicular stomatitis virus.
Moderna and BioNTech/Fosun Pharma/Pfizer are developing mRNA vaccines.
Russia approves the world’s first COVID-19 vaccine, based on human adenovirus
Russia announced that it has approved the world’s first COVID-19 vaccine, which is based on serotypes of human adenovirus, on 11 August.
Experts, however, fear that safety might have been compromised in a rush to approve without completing adequate phase three clinical trials.
Coronavirus vaccines must help to achieve herd immunity, says GlobalData
The level of immunity that the currently being developed COVID-19 vaccines generate is yet to be determined by clinical trials. Apart from being effective, the vaccines need to help in achieving herd immunity to control the pandemic.
The most effective way in which this can be achieved is rapid deployment of the vaccine to the general population, says GlobalData. Vaccinating the global population will require a concerted effort taking into account issues such as non-compliance, cost, and fear of vaccination, GlobalData adds.




