A recent publication from a team of scientists at Trinity College Dublin and University College London demonstrated how gene therapy was able to prevent the degeneration characteristic of retinitis pigmentosa. The study involved the use of 3D retinal organoids that served as templates of human retinal disease and was made using induced pluripotent stem cells and stem cells from patients with retinitis pigmentosa 2 mutations. The retinitis pigmentosa 2 gene provides instructions that are vital for making a protein critical for normal vision. These results emphasise the promise of gene therapies for people with inherited retinal diseases.
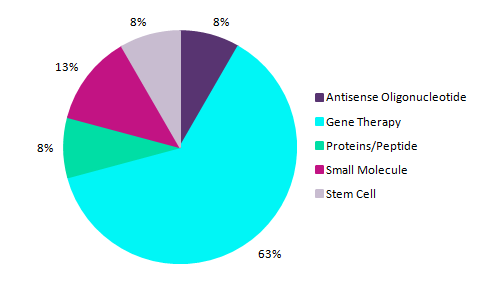
Retinitis pigmentosa is a type of retinal dystrophy. This progressive disease is associated with high levels of clinical and genetic heterogeneity. Replacing the defective gene with a functional one is an approach that has the potential to transform the treatment landscape for such diseases. According to GlobalData’s Intelligence Center, 24 pipeline therapies are currently in different stages of pipeline development (Phase I, Phase II, and Phase III) for the disease in the seven major markets (US, France, Germany, Italy, Spain, UK, and Japan). Of these therapies, 63% are categorised as gene therapies, as shown in Figure 1 above.
In sharp contrast to a large number of pipeline products, there is currently only one marketed therapy, Novartis’ Luxturna (voretigene neparvovec-rzyl). This therapy is only indicated for patients who have a mutation in the retinitis pigmentosa E65 gene. Besides this particular target, other targets are being evaluated among the pipeline therapies, as shown in Figure 2.
Figure 2: Different Targets That Are Currently Being Evaluated Among Gene Therapies in Pipeline Development for Retinitis Pigmentosa

Credit: GlobalData
As these therapies continue to advance to later stages of development, gene therapies are anticipated to remain a dominant feature of the disease’s pipeline, as such targeted therapies are usually associated with few side effects and effective therapeutic outcomes.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataCell & Gene Therapy Coverage on Pharmaceutical Technology supported by Cytiva.
Editorial content is independently produced and follows the highest standards of journalistic integrity. Topic sponsors are not involved in the creation of editorial content.